Predictive Study of Velocimetry in the Coronary Artery after Iodinated Contrast Agent Injection ()
1. Introduction
Monitoring of patients with coronary artery stenosis or having had angioplasty, involves repeated imaging examinations with iodinated contrast agent (ICA) injection. These agents are used to enhance image contrast and to see the diseased arteries, those containing iodine, a heavy chemical element (Z = 53) more clearly [1] . This element confers contrast agents their opacifying properties, justifying their use in medical imaging [2] .
The impact of the use of iodinated contrast agents on the coronary artery is not well understood to date. The increase in the absorbed dose in the coronary artery with ICA has been demonstrated [3] . Work by Hocine et al. (in press) [4] showed a correlation between the increase in the amount of energy deposited in the coronary artery lumen as a function of photon energy and the increase in iodine concentration in the contrast agent.
In order to better understand the potential effect of iodine on the inner wall of the coronary artery, it seemed necessary to study the mechanical aspect of ICA displacement in the artery, their diffusion once injected into the patient and retention time in the coronary artery of ICA. This study will be used to determine artery inner wall exposure time to ICA. Iodine flow velocity and retention time were calculated using the Runge-Kutta 4th order method programmed in C++ and MatLab R2013a language, and the results from our model are discussed below.
2. Equipment and Methods
To calculate flow, it was necessary to model the blood, a complex fluid made up of particles, but also of cells in suspension (red blood cells, white blood cells and platelet) and, the coronary artery with dimensions defined by its length L = 170 mm and inner diameter of 3 mm. Blood was regarded as a Newtonian fluid, meaning it has constant viscosity and induces shear stress. The mean viscosity of blood is considered to be 3 millipascal-second (mPa∙s) or 3 centipoise (cP) and blood density 1070 kg/m3 [5] .
Each iodinated contrast agent has an iodine concentration and viscosity. The concentration maximum is 400 mg of iodine per mL and viscosity is between 6 and 12 cP [2] .
The geometry of healthy coronary arteries and the characteristics of the fluid flowing through them lead us to believe there is a laminar flow pattern with a low Reynolds number.
In an initial approach, flow is modelled in the artery as laminar flow of an incompressible viscous fluid in a duct with rigid wall, with no forks or curves and of constant radius. It is a straightforward model of blood flow in a healthy coronary artery (without artery lumen narrowing).
For the pulsatile flow pattern [6] caused by contractions of the heart, the pressure between the two ends of the artery was modelled by a periodic function. Heart contractions impose a pulsatile pressure gradient in the artery with minimum pressure during the diastole (heart relaxation phase), and maximum pressure during systole (heart contraction phase). A systole and a diastole make up the cardiac cycle and therefore the periodic flow phase.
The pulsatile movements of the blood in the artery caused by heart muscle contraction and relaxation leading to ejection of the blood in the arteries were modelled. Blood flow is considered to be unidirectional, laminar, unstationary and the fluid is considered to be viscous, incompressible and Newtonian. The pressure gradient and velocity vary over time whereas the shape of the conduct (Figure 1) remains unchanged over time.
R is the radius of the conduct, L being its length. We presumed that R is constant over the conduct’s length. P0 represents pressure at one end of the
![]()
Figure 1. Rigid conduct modelling the artery.
artery, and PL that at the other end. The two vectors are orthonormal with a cylindrical base on which the equations are projected.
Blood flow is modelled by the following parameters and equations:
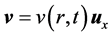 (1)
(1)
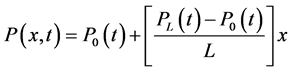 (2)
(2)
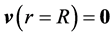 (3)
(3)
The velocity v of a fluid particle is parallel to the axis of the conduct and only depends on its distance from the centre of the conduct and on time (Equation (1)). Pressure varies linearly according to the position in the artery (Equation (2)). Finally, the properties of the viscous fluids set the condition at the following limits: the viscous fluid has zero velocity in the artery’s fixed walls (Equation (3)). Blood flow is governed by the following equations: Navier Stokes equation (Equation (4)) in parallel flow and the incompressibility equation (Equation (5)).
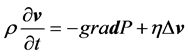 (4)
(4)
 (5)
(5)
where ρ is the density of the fluid expressed in Kg/m3; η is its dynamic viscosity expressed in centipoise (cP) or in Pascal-second (Pas∙s).
The fluid is considered to be incompressible and described by Equation (5).
Equation (6) below gives the expressions for the pressures modelled at the ends of the artery:
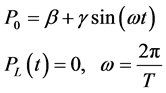 (6)
(6)
ω heart rate and T the cardiac phases (diastolic + systolic).
The pressure gradient is expressed as follows:
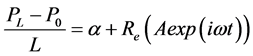 (7)
(7)
where Re is the real part.
The model is linear, and the velocity therefore takes the following form:
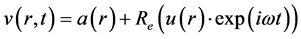 (8)
(8)
where a and u are independent of time; they vary according to radius; u is a complex function.
The Runge-Kutta 4th order method programmed in C++ language in Linux Debian with the compiler’s eMacs produced the values for a(r) and u(r) for various values of the radius of the conduct, ranging from 0 to R, the artery radius.
The Matlab R2013a software was used to calculate the velocity v(r, t) in the artery section according to time.
The Gnuplot graphing software was used to represent the stationary solution in 3D.
The values for a(r) and u(r) were entered into Matlab R2013a again to product v(r, t) for the velocity in the artery section according to different times.
To calculate iodine retention time in the artery, it was necessary to determine the final iodinated blood volume after its dilution.
Iodinated agents are highly viscous products. Viscosity may vary between 6 and 12 cP [6] and according to the molecule properties and their concentration.
When the blood is mixed with iodine, its viscosity increases and may reach 9 cP. However if the iodinated medium is very highly diluted, viscosity remains similar to the viscosity of the blood.
Iodinated blood viscosity and final volume depend on the dilution ratio.
We considered that the viscosity of the blood and contrast agent mixture could be determined by the following relation:
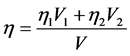 (9)
(9)
where:
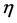 is the viscosity of the mixture;
is the viscosity of the mixture;
 and
and 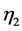 is the viscosity of the blood and
is the viscosity of the blood and
iodine:
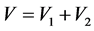
V is the mixture volume.
3. Results and Discussion
3.1. Velocity Profiles and Calculations
Modelling and programming in C++ language produced 2D and 3D blood flow velocity profiles for a heart rate of 80 beats per minute.
Figure 2 shows the resulting 3D profiles for the blood flow stationary velocity solution a(r). In Figure 3 we can see the velocity profiles for the unstationary part resulting on a cardiac cycle. Figure 4 shows the velocity profiles resulting for the full velocity solution and for the pure blood.
Temporal and spatial means were calculated in order to determine the mean
![]()
Figure 2. Blood stationary flow velocity profiles in 3D.
velocity of a fluid particle crossing the coronary artery. Figure 5 shows the mean velocity of a fluid particle over a cardiac cycle. In Figure 5 we note that the blood flow velocity varies between 1.32 m/s and 0.058 m/s. Mean blood velocity with this model is 0.68 m/s. This value is consistent with that of the reference mean coronary flow rate of 250 mL/min [7] , equivalent to a mean blood flow velocity of 0.59 m/s. Therefore the relative deviation with the reference value is 15%.
This blood flow model for coronary artery flows therefore appears to be satisfactory.
The Reynolds number (Re) for this blood flow is:
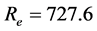
This value is below 2000. It characterises the transition between the laminar and turbulent flow [8] in a rigid, cylindrical conduct.
This result confirms that flow can be modelled by laminar approximation and does not create turbulence in the artery.
The Womersley number for the resulting blood flow is: 5.186.
This number is used to determine the type of forces dominating flow, whether they are inertial or viscous.
A Womersley number of less than 1 is characteristic of flow with which inertial forces are insignificant compared to the viscous forces.
The value obtained is greater than 1, indicating inertial flow.
Figure 6 shows the mean iodinated blood fluid velocity over a cardiac phase. This figure shows the presence of reflux which is characterised by a fleeting inversion of the iodinated blood flow during the systole. We can see in Figure 6 that the main part of the flow takes place during the diastole. This observation is in keeping with the flow characteristics observed and described in literature on coronary artery blood flow [8] . Figure 7 shows flow profiles calculated by the computer model developed in this study for a bolus or single dose of weakly diluted contrast agent with high viscosity.
![]()
Figure 3. Velocity profiles resulting with Matlab resolution for the sinusoidal (pure blood) solution.
![]()
Figure 4. Velocity profiles resulting for the full velocity solution for the pure blood (6 profiles in one cycle).
![]()
Figure 5. Change in mean velocity according to time over a given period.
![]()
Figure 6. Graph showing the mean velocity of the viscous fluid over a given period.
When the contrast agent remains very highly concentrated when flowing through the artery, as during injection for a coronary arteriography, the fluid flowing through the arteries has very high viscosity which is estimated at between 7 and 9 cP. The value of 9 cP is the mean viscosity of contrast agents on the market [2] .
Our calculation shows that the mean velocity of a fluid particle in the artery is 0.29 m/s, with a maximum velocity of 0.67 m/s and minimum velocity of 0.08 m/s in the opposite direction.
The Reynolds number for this blood flow is: 133. The Reynolds number is lower than for pure blood or weakly iodinated blood flow. In the same conditions, viscous flow is therefore slower and has less tendency to present turbulence.
Pulsatile blood flow is characterised by a Womersley number of 3.39. In this case viscous forces play a predominant role in fluid flow.
![]()
Figure 7. Velocity profiles for a blood and iodinated contrast agent mixture.
This study makes us realise that viscosity slows fluid flow in the artery and therefore has an effect on iodinated contrast agent retention time. Therefore, the type of iodinated contrast agent used, route of injection and volume of the product injected can considerably alter the time it takes for the iodinated contrast agent to diffuse in the patient’s body, and especially in coronary arteries.
3.2. Contrast Agent Retention Time
3.2.1. Coronary Artery CT-Scan
Administration of a volume of 80 cm3 iodinated contrast agent in radial infusion (artery or arm) with a flow rate of 4 cm3 per second allows images to be acquired in around 10 s. Bearing in mind that the contrast agent arrives in the arterial blood highly diluted, the viscosity of the blood and iodine mixture is estimated at around 4 cP, value very similar to that of the blood (around 3 cP) and circulating iodinated blood volume is around 480 cm3. According to our model, the contrast agent remains in the artery for less than 2 minutes. For weakly iodinated blood flow, iodinated contrast agent retention time in the artery is estimated at 1 minute and 40 seconds.
3.2.2. Coronary Arteriography
Using our model for coronary arteriography during which the contrast agent is injected directly in the artery, shows that for administration of a 5 mL bolus, travel time in the coronary arteries is estimated at between 2.41 s and 3.61 s. These values were calculated with the hypotheses taken for our model.
Iodinated agent retention time in the artery differs for each patient. The time interval determined allows for a good estimation of contrast agent retention in the coronary arteries. These values were correlated with clinical observations in our radiology department.
3.3. Model Limits
The results presented above were correlated with theoretical values. Our model has some limits however as discussed below. Certain hypotheses were put forward to simplify the complex parameters and to create the model.
The shear stress applied to our fluid, the blood, has an effect on its viscosity and clustering of cells it contains. Higher stress decreases its viscosity. In our model, the variations in blood viscosity with shear stress were set aside.
The dilution ability of iodinated contrast agents (ICA) with blood is still little known and there is also little information on the effect of ICA on the viscosity of the mixture. A linear variation between the viscosity of the mixture and its dilution was assumed in order to simplify consideration of viscosity variations during arterial injection of the iodinated contrast agent.
Coronary artery geometry varies among patients and adapts to patient movement and blood flow changes. Artery walls are deformable and made up of various layers of cells which contract and relax in time with heart contractions. We used a straightforward shape for our model.
Vascular tone, microcirculation and the interaction with endothelial cells increase arterial resistance to blood flow [9] which reduces blood velocity. Blood fluid-artery wall interactions were not discussed.
For modelling pulsatile blood flow in the artery, a heart rate of 80 beats per minute was used as it is the mean heart rate for a healthy adult at rest.
In our model, flow was considered to be parallel. Velocity is parallel to the axis of the conduct over its length, when in actual fact, rough patches on the walls, curves and narrowing of the arteries, along with wall movements, cannot ensure this type of flow. Narrowing of the artery lumen can cause significant turbulence [10] which may have an effect on fluid’s transit time in the conduct.
Our model does not take changes in the surface of diseased coronary arteries into account. They play an important role in blood flow, their roughness and thickening leading to narrowing of the lumen, with a potential effect on blood velocity and blood flow rate in the artery.
It would be useful to study the effect of lumen surface condition and diameter on fluid viscosity and on blood velocity, which may also affect the retention time of the iodinated contrast agents administered.
In this study we created a straightforward model of a complex physiological system with several variable parameters. Fluid/structure interaction and turbulent blood flow caused by uneven walls are difficult to model. These entities are taken as non-linear terms in the equations on fluid progression. Also, given the anatomical differences between patients, a personalised study would be useful and more realistic, taking the various properties of the blood and deformable walls into account, and it would be a way of observing values closest to actual values.
However, the values obtained with our model for calculating the retention time of iodinated contrast agent in the coronary artery for the tests carried out in our hospital are very consistent.
4. Conclusions
Modelling blood flow in coronary arteries was used to estimate iodinated contrast agent retention time in the coronary artery. For coronary artery CT-scans, ICA retention time is 1 minute 40 seconds and for coronary arteriography it is between 2.41 and 3.61 seconds.
The resulting values were compared with theoretical values and with those observed during exploratory tests, and the result is fairly satisfactory and enabled us to validate our model.
The results of this study could be used to better understand the impact of the use of ICA and to better anticipate any effects from their use. These agents, which are sometimes poorly tolerated, may affect certain parameters, blood properties and therefore patients whose arteries are already severely damaged from heavy treatments. Also, the high energy deposited by ionising radiation in the arteries into which these agents are injected, could also carry additional risks. These should be taken into account to ensure that patient exposure to radiation is as low as possible.