An Investigation on Kinetics of Photo Catalysis, Characterization, Antibacterial and Antimitotic Property of Electrochemically Synthesized ZnS and Nano Photocatalysts ()
1. Introduction
Over the past few decades, environmental problems associated with harmful organic pollutants in waste water are driving force for sustained fundamental and applied research in the area of environmental remediation [1] . Semiconductor- assisted photo catalysis has received considerable attention as a promising tool for implementing the purification of waste water and hydrogen energy production [2] . Last two decades have witnessed a rapid advancement in various techniques for the fabrication of nanoparticles [3] [4] . Among the various semiconducting materials, ZnS is a wide-band gap semiconductor of 3.8 eV having luminescent properties and photocatalytic applications. ZnS is one of the II-VI semiconducting material finding application in Cathode ray tubes, IR windows, injection lasers, ultraviolet light-emitting diodes and flat panel displays [5] [6] . Doping of metal ions into ZnS can influence the performance of these photocatalysts. ZnS has been extensively studied with the aim of controlling the size, morphology and crystallinity in order to obtain desired physical properties [7] [8] . Many methods have been used to synthesize ZnS nanoparticles such as sol-gel, hydrothermal, solvothermal and mechanochemical methods. ZnS obtained by this techniques has a wide band gap of 4 - 4.6 eV [9] . An electrochemical procedure based on the dissolution of a metallic anode in a protic solvent, has been used to obtain nanoparticles ranging from 10 to 20 nm with reduced band gap [10] [11] .
Zr is a transition metal mainly used as refractory and opacifier and in small amount as an alloying agent for its strong resistance to corrosion. Zirconium containing compounds are used in many biomedical applications, including dental implants and other restorative practices, knee and hip replacements, although Zr has no known biological role [12] [13] [14] . However, a few papers have been reported on photocatalytic and biological applications of Zirconium doped nanoparticles. Doping of Zr to ZnS is quite attractive, since the dissolution potential of Zr (−1.45 eV) is more negative than Zn (−0.7618 eV) and also the radius of Zr4+ (0.86 Å) is almost similar to that of Zn2+ (0.88 Å). Keeping in view, ZnS and 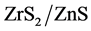 nanocomposites were fabricated by the novel electrochemical method and their catalytic effects on photodegradation of Indigo carmine dye and antibacterial activity using Gram negative bioluminescent Photobacterium leiognathi and antimitotic activity using Allium cepa have been reported here.
nanocomposites were fabricated by the novel electrochemical method and their catalytic effects on photodegradation of Indigo carmine dye and antibacterial activity using Gram negative bioluminescent Photobacterium leiognathi and antimitotic activity using Allium cepa have been reported here.
2. Experimental
2.1. Synthesis of ZnS Nanoparticles
Zinc Sulphide nanoparticles are synthesized electrochemically using Zinc and platinum electrodes as shown in Figure 1. The electrolytic cell consisting of 0.5
![]()
Figure 1. Diagrammatic representation of electrochemical formation of ZnS nanoparticles.
M aqueous Na2S solution and electrodes are separated by 1 cm. The experiment was run for 3 hrs with continuous stirring using 30 mA current and a potential of 20 V. During the electrolysis Zinc electrode acts as anode starts to dissolve and gives Zinc ions which are electrochemically reacted with sulphide ions furnished by Na2S to give ZnS nanoparticles. The obtained nanoparticles are washed repeatedly with distilled water till complete removal of Sodium Sulphide, centrifuged and calcinated at 750˚C for 2 hrs to remove Sodium and hydroxide impurities.
2.2. Synthesis of 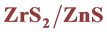 Nanoparticles
Nanoparticles
The experimental set up is similar to synthesis of ZnS. Here Zinc, platinum and Zirconium electrodes were used. The rate of electrochemical reaction is not same for Zr4+ and Zn2+, as the redox potential of Zr4+ and Zn2+ are different. Since the dissolution potential of Zr (−1.45 eV) is more negative than Zn (−0.7618 eV), formation of ZrS2 takes place in competition with ZnS. The mechanism of electrochemical formation is as follows:
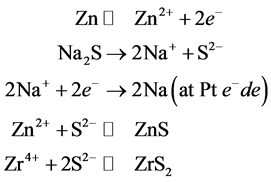
Scheme 1. Mechanism of electrochemical formation of ZrS2/ZnS nanocomposite.
2.3. Determination of Photocatalytic Activities
The photo reactivity of nanocatalysts are influenced by variables such as the dopant, pH of solution, dosage of photo catalyst, concentration of dye and exposure to different source of light viz., sunlight and UV light [15] [16] . Indigo carmine dye (Molecular formula: C16H8O8N2S2, Molecular weight: 466.16, 
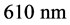 ) solution was prepared by dissolving in distilled water. This solution was used as test contaminant for evaluating photocatalytic activities. To assess the photocatalytic efficiency of the prepared nanoparticles, photodegradation experiments were carried out using different concentration of Indigo carmine dye as substrate and different concentrations of ZnS and
) solution was prepared by dissolving in distilled water. This solution was used as test contaminant for evaluating photocatalytic activities. To assess the photocatalytic efficiency of the prepared nanoparticles, photodegradation experiments were carried out using different concentration of Indigo carmine dye as substrate and different concentrations of ZnS and 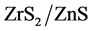 as catalyst. A calculated amount of catalyst was added to the dye solution, stirred in dark for 1 min to establish adsorption/ desorption equilibrium between dye and nanoparticles and then illuminated under 8 W UV source to induce a photoche- mical reaction. Aliquots were taken at an interval of 2 min (in case of ZnS) and 10/15 min (in case of
as catalyst. A calculated amount of catalyst was added to the dye solution, stirred in dark for 1 min to establish adsorption/ desorption equilibrium between dye and nanoparticles and then illuminated under 8 W UV source to induce a photoche- mical reaction. Aliquots were taken at an interval of 2 min (in case of ZnS) and 10/15 min (in case of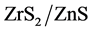 ) and percent transmittance was determined.
) and percent transmittance was determined.
The adsorption and photocatalytic conversion (g%) was calculated as follows [17] :
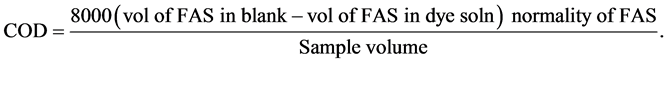
The mineralization of dye was measured by the decrease of chemical oxygen demand (COD) of the solution. The COD was measured according to the standard dichromate titration method [18] [19] . The mineralization efficiency of dye was estimated by the following expression:
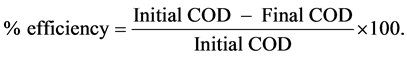
The decrease in COD (mg/l) and increase in % T of the dye solution with colour removal was observed as follows:
 .
.
The mechanism of photodegradation can be represented as follows [20] [21] .
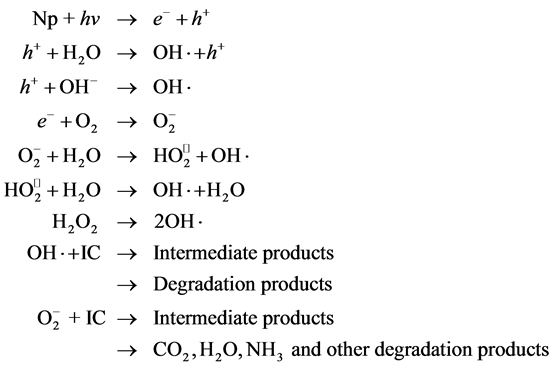
Scheme 2. Mechanism of dye degradation by OH radical.
3. Results and Discussion
3.1. X-Ray Diffraction
The XRD pattern of synthesized ZnS and  nanoparticles are shown in Figure 2. The XRD for ZnS shows three main diffraction peaks at 2θ values 31.61, 34.28 and 36.08. The obtained peak positions correspond to Zinc blended type patterns and the XRD pattern is well matched with standard cubic ZnS [6] . The XRD of ZrS2/ZnS nanoparticles are compared with ZnS nanoparticles and are as follows. From the XRD data it is evident that no much change in the position of diffraction peaks were observed compared to ZnS nanoparticles. A possible reason could be that Zr4+ entered into the crystal lattice of ZnS and suppress the growth of ZnS crystals, because radius of Zr4+ (0.86 Å) smaller than that of Zn2+ (0.88 Å) and Zr substitutes Zn in the lattice. The slight change of lattice parameters of
nanoparticles are shown in Figure 2. The XRD for ZnS shows three main diffraction peaks at 2θ values 31.61, 34.28 and 36.08. The obtained peak positions correspond to Zinc blended type patterns and the XRD pattern is well matched with standard cubic ZnS [6] . The XRD of ZrS2/ZnS nanoparticles are compared with ZnS nanoparticles and are as follows. From the XRD data it is evident that no much change in the position of diffraction peaks were observed compared to ZnS nanoparticles. A possible reason could be that Zr4+ entered into the crystal lattice of ZnS and suppress the growth of ZnS crystals, because radius of Zr4+ (0.86 Å) smaller than that of Zn2+ (0.88 Å) and Zr substitutes Zn in the lattice. The slight change of lattice parameters of  also proved that the Zr ions were incorporated into the ZnS lattice.
also proved that the Zr ions were incorporated into the ZnS lattice.
From the XRD data the cell parameters are calculated for ZnS nanoparticles and it is found to be a = b ≠ c (a = 8.483 Å, b = 8.483 Å and c = 9.957 Å) and
![]()
Figure 2. XRD patterns of ZnS and ZrS2/ZnS nanoparticles.
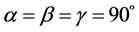 . Accordingly, ZnS nanoparticles belong to Tetragonal crystal system. Using XRD data, the cell parameters calculated for
. Accordingly, ZnS nanoparticles belong to Tetragonal crystal system. Using XRD data, the cell parameters calculated for 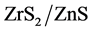 nanoparticles are found to be a = b ≠ c (a = 6.900 Å, b = 6.900 Å and c = 9.806 Å) and
nanoparticles are found to be a = b ≠ c (a = 6.900 Å, b = 6.900 Å and c = 9.806 Å) and![]() . Accordingly,
. Accordingly, ![]() nanoparticles belong to Tetragonal crystal system. The average crystallite size was calculated using Williamson-Hall plot [22] and it was found to be 57.2 nm for ZnS and 43.8 nm for
nanoparticles belong to Tetragonal crystal system. The average crystallite size was calculated using Williamson-Hall plot [22] and it was found to be 57.2 nm for ZnS and 43.8 nm for ![]() nanoparticles. The angle strain is 3.46 × 10−2 for ZnS and 7.59 × 10−4 for
nanoparticles. The angle strain is 3.46 × 10−2 for ZnS and 7.59 × 10−4 for ![]() nanocomposite.
nanocomposite.
3.2. Optical Absorption Spectra
The UV-Visible spectrum of ZnS and ![]() nanoparticles (Figure 3) over the range 200 - 600 nm showed that the synthesized nanoparticles are photoactive under UV light radiation. ZnS nanoparticles showed three absorption peaks where as
nanoparticles (Figure 3) over the range 200 - 600 nm showed that the synthesized nanoparticles are photoactive under UV light radiation. ZnS nanoparticles showed three absorption peaks where as ![]() nanoparticles showed two peaks in the UV region. There is no absorption peak in the visible region. The band gaps of the samples are calculated using Tauc’s plot [23] [24] and was found to be 2.7 eV for ZnS and 3.9 eV for
nanoparticles showed two peaks in the UV region. There is no absorption peak in the visible region. The band gaps of the samples are calculated using Tauc’s plot [23] [24] and was found to be 2.7 eV for ZnS and 3.9 eV for ![]() nanoparticles as shown in Figure 4. The obtained band gap value of ZnS nanoparticle is lower than that of the bulk value of ZnS (3.68 eV). This blue shift of the band gap of ZnS nanoparticle occurs due to quantum confinement effect [25] . The band gap of
nanoparticles as shown in Figure 4. The obtained band gap value of ZnS nanoparticle is lower than that of the bulk value of ZnS (3.68 eV). This blue shift of the band gap of ZnS nanoparticle occurs due to quantum confinement effect [25] . The band gap of ![]() is very much higher than
is very much higher than
that of ZnS. This supports lower photocatalytic efficiency of ![]() nano
nano
particles compared to ZnS nanoparticles.
![]()
Figure 3. UV-Visible spectra of (a) ZnS and (b) ZrS2/ZnS nanoparticles.
![]()
![]()
Figure 4. Tauc’s plot of ZnS and ZrS2/ZnS nanoparticles.
3.3. EDX of ZnS and ![]() Nanoparticles
Nanoparticles
The EDAX analysis confirmed the presence of Zinc and Sulphur in ZnS and Zirconium, Zinc and Sulphur in ![]() nanoparticles (Figure 5 and Figure 6). The surface morphology of ZnS and
nanoparticles (Figure 5 and Figure 6). The surface morphology of ZnS and ![]() nanoparticles was observed by FE-SEM analysis. The SEM images showed that the synthesized nanoparticles consisted of agglomerated particles (Figure 7).
nanoparticles was observed by FE-SEM analysis. The SEM images showed that the synthesized nanoparticles consisted of agglomerated particles (Figure 7).
![]()
Figure 6. EDAX for ZrS2/ZnS nanoparticles.
3.4. Photo Catalytic Degradation of Indigo Carmine Dye and COD Measurements
3.4.1. Effect of Concentration of Dye
To ensure the optimum dye concentration, photodegradation is carried out with different concentration of Indigo carmine with constant weight of catalyst (Table 1 and Figure 8). As the optimum concentration of catalyst for ZnS nano particle is 0.02 g, keeping this as standard the same amount of ![]() nanopartilce is taken for comparision in the further work. Beyond the optimum dye concentration, as the initial concentration of dye increases, the degradation efficiency reduces. The possible reason is that, as initial concentration of dye is increased, more dye molecules are adsorbed onto the surface of the catalyst. But the adsorbed dye molecules are not degraded immediately because the intensity of the light and the amount of catalyst is constant and also the light penetration is less. Also with increase in dye concentration, the solution becomes more intense coloured and the path length of the photons entering the solution is decreased thereby fewer photons reached the catalyst surface [17] . Hence there will be reduction in the production of ROS species like hydroxyl and superoxide
nanopartilce is taken for comparision in the further work. Beyond the optimum dye concentration, as the initial concentration of dye increases, the degradation efficiency reduces. The possible reason is that, as initial concentration of dye is increased, more dye molecules are adsorbed onto the surface of the catalyst. But the adsorbed dye molecules are not degraded immediately because the intensity of the light and the amount of catalyst is constant and also the light penetration is less. Also with increase in dye concentration, the solution becomes more intense coloured and the path length of the photons entering the solution is decreased thereby fewer photons reached the catalyst surface [17] . Hence there will be reduction in the production of ROS species like hydroxyl and superoxide
![]() (a)
(a)![]() (b)
(b)
Figure 7. SEM micrographs of (a) ZnS and (b) ZrS2/ZnS nanoparticles.
radicals [26] .
3.4.2. Effect of Catalyst Loading
The experiments were performed by taking different amount of catalyst varrying from 0.02 to 0.06 g in order to study the effect of catalyst loading (Table 2 and Figure 9). Photocatalytic rate initially increases with catalyst loading and then decreases at high values because of light scattering and screening effects [17] [27] . The tendency toward agglomeration also increases at high solid concen- tration, resulting in a reduction in the surface area available for light absorption and a decrease in photocatalytic degradation rate. The number of active sites in
![]()
Table 1. Effect of concentration of dye on the rate of degradation.
![]()
Table 2. Effect of catalyst loading on the rate of photo-degradation.
solution will increase with catalyst loading, a point appears to be reached where light penetration is compromised because of excessive particle concentration [28] . A further increase in catalyst loading beyond the optimum will result in non-uniform light intensity distribution, so that the reaction rate would indeed be lower with increased catalyst dosage.
3.4.3. Effect of Temperature
Temperature is one of the important factor which effects the rate of photo- degradation. Increase of temperature is an indication of slighter increase in the rate of photodegradation as raise in temperature results in number of effective collisions leading to higher rate of reaction. However, the photodegradation efficiency is not much affected (Table 3 and Figure 10).
3.4.4. Reuse of Catalyst
The possibility of reusing the photocatalyst was tested to see the cost effec- tiveness of the method used. After degradation of the dye, the dye solution was kept overnight and then the supernatant liquid was decanted. The photocatalyst was thoroughly washed with double distilled water and then reused for the photodegradation by taking fresh IC dye solution. The reuse sample has shown almost same degradation efficiency compared to the fresh samples (Table 4 and
![]()
Figure 8. Plot of log % T vs. time with respect to different initial concentration of dye and COD values in case of (a) ZnS and (b) ZrS2/ZnS nanoparticles.
![]()
Figure 9. Plot of log % T vs. time with respect to catalyst loading and COD values in case of (a) ZnS and (b) ZrS2/ZnS nanoparticles.
![]()
Figure 10. Plot of log % T vs. time with respect to temperature in case of (a) ZnS and (b) ZrS2/ZnS nanoparticles.
![]()
Table 3. Effect of temperature on degradation of dye.
![]()
Table 4. Efficiency of catalyst in second use.
Figure 11), while an obviously decrease in photoactivity was noticed with the reuse cycles [29] . This indicates the nano samples can be regenerated and reused
![]()
Figure 11. Plot of log % T vs. time with respect to reuse of catalyst and COD values in case of ZnS and ZrS2/ZnS nanoparticles.
with very low or significance change in the efficiency. An obviously decrease in rate of reaction was observed with the second use of catalyst. Reuse cycles might cause the aggregation of photocatalyst and the decrease in specific surface area and the losses of catalyst, resulting in a loss of catalytic activity [30] .
4. Biological Activities
4.1. Antibacterial Activity
4.1.1. Bacterial Growth Condition
The Gram negative bioluminescent Photobacterium leiognathi (accession number: KM434234), isolated from coastal area of Goa, were used in this study. Bacteria were grown in nutrient broth (NB) containing 3% sodium chloride with aeration at 25˚C for 24 h. 10 μl of the overnight culture was inoculated into 100 ml of nutrient broth and incubated under same condition until the OD600 reaches 0.5 (approximately 12 hours) as at this OD bacteria were emitting the maximum amount of light.
4.1.2. Reagents
1 mg/ml stock solution nanoparticle (NPs) was prepared in sterile distilled water. To disperse the NPs, the suspension was sonicated before use. Later NPs dilutions were prepared in sterile broth. As these solutions are later inoculated with an equal amount of bacteria in broth, the dilutions are prepared at concentration twice the desired final concentration.
4.1.3. Minimum Inhibitory Concentration (MIC) Assay
Broth microdilution technique has been used to determine the MIC of nanoparticle. For this purpose, two fold serial dilutions of the compound ranging from 500 to 0.4 mg/ml were performed in 96-well white microtiter plate. Initially 100 μl of bacterial inoculums was placed into the wells of the plate and later each well seeded with 100 μl of nanoparticle dilutions. Bacterial luminescent intensity was measured using a luminometer after 6 h incubation at 25˚C with 150 rpm shaking. In addition to the NP-treated well, each plate had untreated bacterial culture as control. Sterile broth containing NP and sterile broth also served as blank for the test and control respectively. NP efficacy was calculated from the blanks, control and treated luminescence values on a plate.
Percentage of inhibition = ![]()
where ![]() denotes the average luminescence for sterile broth,
denotes the average luminescence for sterile broth, ![]() denotes the average luminescence for sterile broth containing NP, C denotes the average luminescence for control and T denotes the average luminescence for treated wells.
denotes the average luminescence for sterile broth containing NP, C denotes the average luminescence for control and T denotes the average luminescence for treated wells.
4.1.4. Result and Discussion
The MIC is defined as the lowest concentration of NPs that inhibits the growth of a microorganism. The percentage of inhibition for ZnS and ![]() has showed in Table 5 and Figure 12. The MIC value for ZnS and
has showed in Table 5 and Figure 12. The MIC value for ZnS and ![]() is 500 and 15.6 μg/ ml respectively. These results indicate that the
is 500 and 15.6 μg/ ml respectively. These results indicate that the ![]() has higher antimicrobial activity than zinc sulphide.
has higher antimicrobial activity than zinc sulphide.
4.2. Antimitotic Activity
The antimitotic activities of synthesized nanoparticles were determined by onion root tip method. Allium cepa has been used to evaluate the antimitotic activity of synthesized ZnS and ![]() nanoparticles. The results of antimitotic activity are given in Table 6 and the percentage inhibition of cell division by ZnS and
nanoparticles. The results of antimitotic activity are given in Table 6 and the percentage inhibition of cell division by ZnS and ![]() nanoparticles comparative to control is given in Figures 13(a)-(d). Onion roots in ZnS and
nanoparticles comparative to control is given in Figures 13(a)-(d). Onion roots in ZnS and ![]() of concentration (25, 50 and 75 ppm)
of concentration (25, 50 and 75 ppm)
![]()
Table 5. Inhibition of bacterial growth at different concentrations of nanoparticles (mg/ ml).
![]()
Figure 12. Plot of % Inhibition vs. Conc. in μg/ml with respect to ZnS and ZrS2/ZnS Nanoparticles.
![]()
Table 6. Antimitotic activity results of ZnS and ZrS2/ZnS nanoparticles.
at different time duration (12, 18 and 24 hrs) exhibited changes in chromosomes and shape of the cells with elongated appearance. The change in chromosomes and cellular morphology were achieved in increasing time and concentration. ZnS and ![]() nanoparticles showed good inhibitory effect by inhibiting the cell growth.
nanoparticles showed good inhibitory effect by inhibiting the cell growth.
From the above observations, the partial-c-mitosis, full-c-mitosis with partial functional spindles and comparatively normal mitotic cells phases were noticed in various cells of the same root tip between 12 - 24 hrs time duration. Therefore, the antimitotic ability of ZnS and ![]() was remarkable in controlling the cell division and acts as potent antimitotic agents.
was remarkable in controlling the cell division and acts as potent antimitotic agents.
5. Conclusion
In this study, we have reported the synthesis of ZnS and ![]() nanoparticles by novel electrochemical method which is simple, cost effective and eco- friendly method. The photo degradation by these semiconductors offers a green technology for the removal of hazardous compounds present in waste water and industrial effluents. The kinetics of degradation of Indigo carmine has been studied. The complete degradation reaction was confirmed by conducting COD experiment. The synthesized nanoparticles are capable of entering into the Allium cepa cell and bacterial cell, therefore, inhibit the cell growth and hence confirm the biological activity as a potent antimitotic and antimicrobial agents. Moreover,
nanoparticles by novel electrochemical method which is simple, cost effective and eco- friendly method. The photo degradation by these semiconductors offers a green technology for the removal of hazardous compounds present in waste water and industrial effluents. The kinetics of degradation of Indigo carmine has been studied. The complete degradation reaction was confirmed by conducting COD experiment. The synthesized nanoparticles are capable of entering into the Allium cepa cell and bacterial cell, therefore, inhibit the cell growth and hence confirm the biological activity as a potent antimitotic and antimicrobial agents. Moreover, ![]() nanoparticles have less photo catalytic activity compared with ZnS nanoparticles towards photodegradation. The presence of Zr suppresses the rate of photo-catalytic activity. ZnS nanoparticles are very good photocatalyst compared with
nanoparticles have less photo catalytic activity compared with ZnS nanoparticles towards photodegradation. The presence of Zr suppresses the rate of photo-catalytic activity. ZnS nanoparticles are very good photocatalyst compared with ![]() nanocomposite. Hence,
nanocomposite. Hence, ![]() nanocomposite can be used to block UV radiation and can be used in the preparation of cosmetics. The degradation efficiency is ~95% in case of ZnS where as 83% in
nanocomposite can be used to block UV radiation and can be used in the preparation of cosmetics. The degradation efficiency is ~95% in case of ZnS where as 83% in ![]() nanocomposites.
nanocomposites.
Acknowledgements
This work has been supported by University of Mysore, Mysuru and the Author Uma, H. B. is thankful to UGC, New Delhi for providing financial assistance through BSR meritorious fellowship.
Competing Interests
Authors have declared that no competing interests exist.