Evaluation of Antioxidant, Antiglycant and ACE-Inhibitory Activity in Enzymatic Hydrolysates of α-Lactalbumin ()
1. Introduction
Milk whey proteins are low cost by-products of the cheese making industry known for their high nutritional value. Whey stands for a 90% of bovine milk containing 80% of caseins and 20% of whey proteins respected to total milk protein content [1] . Whey proteins consist of two major proteins: β-lactoglobulin (58% w/w of whey proteins) and α-lactalbumin (20% w/w of whey proteins) [2] . Cheese manufacture produces near 86 million tonnes of whey proteins per year [3] having a big impact on the environment and representing a huge waste of proteins with high potential uses.
Food industry has invested in the revalorization of its effluents to create added-value products by investigating their biologically beneficial properties [4] . α-lactalbumin is the second major protein of whey proteins which has a globular structure with 123 residues and two subdomains (α with 4-helices and β with β-sheets and loop regions) [5] ; [6] , a molecular weight of 14 - 17 kDa and an isoelectric point of 4.2 [6] , four disulfide bonds and a calcium ion [7] . In the primary structure of these protein tryptophan, leucine and cysteine can be found and these residues could be the responsible ones for great antioxidant properties. α-lactalbumin has 8 cysteines which stabilize its tertiary structure through disulfide bonds [8] . α-lactalbumin has a great amount of essential amino acids [8] , most of which could confer bioactive properties by being released as peptides after enzymatic hydrolysis [8] [9] . These bioactive properties would be antioxidant, antiglycant and antihypertensive, among others [9] .
Antioxidants are compounds which neutralize oxygen and nitrogen reactive species produced by cells in oxidative stress conditions [10] . Oxidative stress is caused by normal cellular metabolism of aerobic organisms in which these species are generated, including free radicals (atoms, group of atoms or molecules which at least have one unpaired electron) [11] , in a higher quantity than what the cell can overcome by its endogen mechanisms (superoxide dismutase, catalase and glutathione peroxidase) [10] ; [12] . Glutathione is a non proteic molecule of 3 residues which takes part in endogen response to oxidative stress to prevent chronic diseases. Oxygen reactive species and oxidative stress are involved in chronic diseases such as cancer, diabetes, ischemia, cardiovascular diseases, infection, Parkinsonism, atherosclerosis and arthritis, among others [10] [13] . Sadat et al. [14] studied antioxidant activity of the peptides obtained from the hydrolysis of α-lactalbumin with termolysine, being able to isolate and identify the peptides responsible for these activity; residues of major activity were tryptophan and tyrosine positioned at the end of different peptides. α-lactalbumin beholds more aromatic residues than β-lactoglobulin (whey protein in greater proportion) [15] .
One major chronic disease is diabetes which consists of a hyperglycemia due to relative or absolute deficiency of insulin and/or resistance to it [16] [17] . Diabetes is the forth cause of death in developed countries and is an epidemic in a lot of developing ones. It is estimated that this disease affects 25% of world population [18] . Glycation is the major cause of spontaneous damage to proteins in physiologic systems being exacerbated in diabetes. Advanced glycation end products (AGEs) formation is reached by modification of N-terminus groups and residues such as lysine, cysteine and arginine [19] [20] . Protein glycation is produced by Maillard reaction (nonenzymatic browning reaction) which starts with the condensation between an amino group available and a carbonyl, in general, a reducing carbohydrate to form a Shiff base. These Shiff base suffers chemical rearrangements forming Amadori or early glycation products [21] [22] [23] . When these products accumulate crosslinked proteins can be formed [21] . AGEs can also be generated by glucose autooxidation and lipids peroxidation through oxidative stress, forming dicarbonyl derivatives (α-oxaldehides such as methylglyoxal (MGO), glyoxal and 3-deoxyglucosone) which can interact with monoacids to produce AGEs [21] [24] [25] . This would imply that the presence of antioxidants in foods could be related to a reduction in AGEs formation (antiglycant activity) [24] [25] [26] .
Another chronic disease is cardiovascular disease which is associated with hypertension (arterial blood pressure sustained elevation) and is of great incidence in developed countries [27] . The dipeptidyl carboxypeptidase ACE (angiotensin I-converting enzyme) regulates arterial pressure by the renin-angiotensin system for which ACE inhibition reduces arterial pressure by reducing the levels of angiotensin II (vasoconstrictor) and increasing bradykinin (vasodilator) [27] . Captopril and enalapril are reference drugs to treat hypertension but they have secondary effects for which foods could avoid [28] . Some authors have found antihypertensive properties in milk proteins [9] [29] enhancing ACE inhibition by enzymatic hydrolysis [15] [30] because of the formation of tripeptides.
In the case of α-lactalbumin, it has not been thoroughly investigated in terms of obtaining powerful antioxidant peptides from enzymatic hydrolysis [14] as well as antiglycant and ACE-inhibitory activity.
Thus, the objective of this study was to evaluate the effect of different conditions of enzymatic hydrolysis (enzyme:susbstrate ratio and time of reaction) on antioxidant activity, using a response surface methodology (RSM), and hydrolysates characterization. In addition, ACE-inhibition and antiglycant activity were evaluated on the hydrolysate that presented the highest antioxidant activity.
2. Materials and Methods
2.1. Materials
Protein isolate of α-lactalbumin (Biopure-lactoalbuminTM) was provided by Davisco Food International Inc. (Le Sueur, MN, USA). Alcalase was from Novozymes Biopharma US Inc. Buffer salts were NaHPO4 (Mallinckrodt) and NaH2PO4 (J. T. Baker). Folin reagent and 1-anilin-8-naftensulfide (ANS) were purchased from Sigma Aldrich (St. Louis, MO). For electrophoresis analysis: acrylamide, bis-acrylamide, tricine, sodium dodecyl sulphate (SDS) and Tris were purchased from MP Biomedicals; 2-mer- captoethanol and the molecular weight marker (MWM) with 7 bands from 2.5 to 17 kDa (MW-SDS-17S) were purchased from Sigma Aldrich (St. Louis, MO); bromophenol blue (J. T. Baker), HCl (Dorwill), acetic acid (VETEC). For ABTS assays: 2,20- azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) and 6-hy- droxy-2,5,7,8-tetramethylchroman-2-acid (Trolox) were purchased from Sigma Aldrich, and potassium persulphate was from J. T. Baker. For ORAC (Oxygen Radical Antioxidant Capacity)-FL assay: Fluorescein (FL) disodium salt and 2,20-azobis (2- methylpropionamidine) dihydrochloride (AAPH) were obtained from Sigma Aldrich (St. Louis, MO). For antiglycant assays: methylglyoxal (MGO) and aminoguanidine (AG) were purchased from Sigma Aldrich (St. Louis, MO); bovine serum albumin (BSA) (GIBCO BRL) and glucose (Glc) (Fluka). For angiotensin converting enzyme (ACE)-inhibition assay: histidil-hipuril-leucine (HHL) was purchased from Sigma Aldrich (St. Louis, MO).
2.2. α-Lactalbuminisolatehydrolysis
The hydrolysis reaction was carried out using Alcalase enzyme with 8% (w/V) of protein isolate in buffer phosphate solution 100 mM pH 7 and incubation temperature of water bath 30˚C with agitation of 150 rpm. After incubation time (as in Section 2.3), reaction was stopped by heating at 100˚C for 10 minutes. Samples were freezed, lyophilized and stored at −20˚C for subsequent analysis.
2.3. Experimental Design
For the optimization of hydrolysis process an experimental design [31] (compose central design) was used based on a response surface model, full factorial design. The hydrolysis reaction was evaluated by determining ABTS and ORAC-FL values (response variables) with the variation of two factors, enzyme:substrate ratio (r) (% w/w) and time (t) (minutes). Seven samples were prepared: four assays, two factors (r and t) with two levels (0.0050 and 0.1000% w/w, 0 and 60 minutes, respectively) and three central points (0.0525% w/w and 30 minutes, for r and t factors, respectively) in order to estimate the experimental error (sample conditions are listed in Table 1). The equation for the proposed model of response variables ABTS and ORAC-FL (Yi) is shown in Equation (1):
 (1)
(1)
where β0 is the intersection point; β1 and β2 are de linear coefficients; β1,2 is the coefficient which represents the interaction between the independent variables (factors r and t); and ε is the variable error. Model parameters where calculated with Statgraphic Plus version 5.1 program by multiple linear regression (MLR).
2.4. Characterization of α-Lactalbumin Hydrolysates
Protein content was determined by Lowry method [32] preparing 0.3 mg/mL solutions of each hydrolysate in phosphate buffer 10 mM pH = 7.4. Surface hydrophobicity was determined with the method described by Hayakawa and Nakai [33] , based in spectrofluorimetry measurements with ANS (fluorescent probe). Hydrolysates solutions of 1 mg/mL were prepared in sodium phosphate buffer 100 mM pH = 7.0, agitated for 30 minutes at room temperature and centrifuged at 10.000 g and 4˚C for 10 minutes. 8 mM
![]()
Table 1. Results of protein percentage, surface hydrophobicity and hydrolysis percentage of the α-lactalbumin hydrolysates.
Results are expressed as the means ± SD (n = 6). ANOVA analysis was made by column using Tukey test. Means values in the same column with different letters (a, b, c) state significant differences (p < 0.05).
ANS solution was prepared weighting 0.00128 g of ANS in 5 mL of buffer. Measurements were made in spectrofluorimeter microplate reader (Varioskan® Flash, Thermo Electron Corporation) by adding 250 μL of different concentration solutions and 3.75 μL of ANS in each well. Also, 250 μL of MeOH and 3.75 μL of ANS were added to determine maximum fluorescence. This probe was measured at 363 nm and 475 nm wave lengths of excitation and emission, respectively. According to Kato and Nakai [34] surface hydrophobicity index (S0) corresponds to the initial slope of the curve of probe’s fluorescence intensity as a function of protein concentration. Equation (2) shows how to calculate surface hydrophobicity:
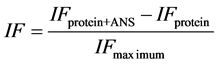 (2)
(2)
where IFprotein+ANS is the protein’s fluorescence intensity with ANS, IFprotein is protein’s fluorescence intensity without ANS, IFmaximum is the maximum fluorescence which corresponds to methanol with ANS.
Tricine-SDS-PAGE analyses were performed for the hydrolysates according to the method described by Schägger and von Jagow [35] . Electrophoresis gel contains three parts: stacking, spacer and separating gels with 4%, 10% and 16% of acrylamide, respectively. Sample solutions were prepared of 2 mg/mL in sample buffer containing 12% glycerol, 4% SDS, 2% 2-mercaptoethanol (v/v), 50 mMTris, 0.01% bromophenol blue and 0.1 M HCl for pH adjustment. Cathodic buffer was prepared with 100 mMTris, 0.1% SDS (w/V) and 100 mMTricine, pH 8.9. Anodic buffer was prepared with 200 mMTris and 0.1 M HCl for pH adjustment (pH 8.9). Electrophoresis conditions were as follows: 30 V and 30 mA until the front went through the stacking gel and then 90 V (Sigma Aldrich PS 250-2) for two gels of 1.0 mm thick. The apparatus used was an Amersham Biosciences SE 260. Gel fixation was performed by 50% methanol and 10% acetic acid solution during 30 minutes agitation and coloration was performed by a Coomasie Blue R250 0.1% solution in 30% methanol and 10% acetic acid over night. Decoloration was performed by 30% methanol and 10% acetic acid solution during 30 minutes agitation and several washes with 15% methanol and 5% acetic acid solution. Molecular weight marker with 7 bands from 2.5 to 17 kDa was used.
SE-HPLC analysis were carried out as described by Molina et al. [36] by the equipment Shimadzu, SPD-20A detector and LC-10AT pump detecting at 280 nm. Samples were eluted in a Molecular Exclusion Column BioSep-Sec 2000 with an isocratic flow of 1 mL/min and phosphate buffer 50 mM (pH 6.8) 0.5% of SDS as mobile fase. Samples were prepared in mobile fase in a concentration of 2 mg/mL. Hydrolysis percentage was determined by quantifying α-lactalbumin in each hydrolysate with anα-lactalbumin calibration curve.
2.5. Antioxidant Capacity
Antioxidant capacity was determined by two methods: ABTS and ORAC-FL, as electron transfer (ET) and hydrogen atom transfer (HAT) methods, respectively. ABTS was performed by the method described by Re et al. [37] in which antioxidant capacity is determined by measuring ABTS reagent absorbance at 734 nm. All samples were compared with a calibration curve of trolox. Briefly, ABTS stock solution (7 mM) with potassium persulphate (2.45 mM) was mixed with phosphate buffer 5 mM pH 7.4 until reaching an absorbance of 0.7. Then, 30 μL of sample was mixed in tubes with 3 mL of ABTS in buffer and 10 minutes later absorbance was measured in a spectrophotometer. In addition, doses-response curves were done to calculate IC50 by constructing the curve% Inhibition vs. [Protein] (mg/mL) (from 0.25 to 5 mg/mL of protein) and obtaining the logarithmic function in order to calculate the corresponding protein concentration to 50% of inhibition. Inhibition percentage is calculated according to Equation (3):
 (3)
(3)
where Acontrol is the absorbance of 3 mL of ABTS in buffer with 30 μL of buffer and Aantioxidant is the absorbance of 3 mL of ABTS in buffer with 30 μL of trolox or sample.
ORAC-FL was performed as described by Ou et al. [38] modified by Dávalos et al. [39] in which a fluorescent probe (FL) is oxidized by an oxygen radical generator (AAPH) lowering its fluorescence intensity. Measurements were displayed at 485 nm and 520 nm of excitation and emission wave lengths, respectively, at 37˚C for 104 minutes in the equipment Varioskan® Flash. Briefly, samples and reagents were prepared in phosphate buffer 75 mM pH 7.4. Each well had a final volume of 200 μL: 120 μL of 1.17 mM fluorescein solution (70 nM final concentration), 60 μL of AAPH (12 mM final concentration) and 20 μL of antioxidant substance (trolox or sample). All samples were prepared in duplicate and each one of the preparations was tested at least in triplicate. Equation (4) shows calculation of the area under the curve (AUC) Fluorescence vs Time:
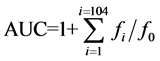 (4)
(4)
where f0 is the fluorescence at 10 minutes of incubation at 37˚C and fi is the fluorescence measured every minute. Curves of Fluorescence vs Time were normalized to the curve of the blank calculating the net AUC as the difference between AUCantioxidant (trolox or sample) and AUCblank. Trolox calibration curve (net AUCtrolox vs nTE (μmol TE)) was constructed in order to calculate samples antioxidant capacity (μmol TE/mg of protein). Besides punctual measurements, IC50 values were obtained by constructing the curve nTE vs [Protein] (mg/mL) to obtain logarithmic function and the concentration of protein correspondent to 50% inhibition of peroxyl radicals.
2.6. Antiglycant Activity
Antiglycant activity was assessed using two methods: BSA-MGO and BSA-Glc.
In vitro glycation assay with BSA-MGO was determined as described by Wang et al. [28] with some modifications. Briefly, concentrations of 5 and 10 mM (final concentration) of methylglyoxal (MGO) were mixed with 2 mg/mL BSA (1 mg/mL final concentration) and sufficient volume of phosphate buffer 10 mM pH 7.4 with 0.02% sodium azide, in a final volume of 5 mL. Negative control was prepared with 2.5 mL of BSA and 2.5 mL of phosphate buffer, and positive control was prepared with BSA, MGO and sufficient volume of phosphate buffer. Aminoguanidine was used as the reference substance and it was mixed with BSA, MGO and phosphate buffer in three final concentrations: 1, 4 and 8 mM. Sample 4 was tested in concentrations of 1 and 10 mg/mL. All the tubes were incubated at 37˚C for 13 days measuring fluorescence at 340 nm and 420 nm of excitation and emission wave lengths, respectively. Inhibition percentage was calculated as fallows (Equation (5)):
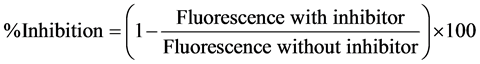 (5)
(5)
In vitro glycation assay with BSA-Glc was determined as described by Mesías et al. (25) with some modifications. Glucose was prepared in concentrations of 20 and 40 mM (final concentration) in a final volume of 5 mL mixing it with BSA (1 mg/mL final concentration) and sufficient volume of the same phosphate buffer as BSA-MGO model. Negative control used was the same as BSA-MGO assay and positive control was prepared with Glc, BSA and phosphate buffer. Sample tested concentrations were the same, incubation and measurement conditions were the same as well.
2.7. Antihypertensive Activity
Antihypertensive activity was determined as described by Cushman & Cheung [40] modified by Kim et al. [41] . In this assay, the inhibition of angiotensin converting enzyme (ACE) was evaluated. Briefly, samples concentration of 5 mg/mL were prepared in borate buffer 0.2 M pH 8.3 and agitated at 25˚C, 700 rpm, for 30 minutes to solubilize them. Then, samples were centrifuged at 9.500 rpm for 20 minutes at 20˚C. Assay buffer (borate buffer 0.2 M and NaCl 2M, pH 8.3) was added to the sample, mili Q water and HHL 5 mM. Eppendorf tubes were incubated at 37˚C, 700 rpm, for 5 minutes, and ACE was added in cold (0 - 10 mU). Same tubes were incubated at 37˚C, 700 rpm, for 30 minutes and after, tubes were incubated at 90˚C for 10 minutes. Finally, colour reagent and potassium phosphate buffer 0.2 M pH 8.3 were added, samples were centrifuged at 20˚C, 6000 rpm, for 10 minutes and supernatant absorbance was measured at 382 nm. ACE inhibition percentage was calculated according to equation 6:
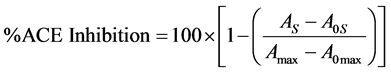 (6)
(6)
where As and A0s are the absorbance of sample in ACE’s presence and absence, respectively; Amax is the absorbance in the absence of sample and A0max is the absorbance in the absence of sample and ACE.
2.8. Statistical Analysis.
All the measurements were determined at least in triplicate. Results were expressed as means values ± standard deviation. Analysis of variance (ANOVA) and Tukey test were applied to determine significant differences between values (p < 0.05). Statistical analysis was done using Infostat v. 2015 and Statgraphic Plus v. 5.1 programs.
3. Results and Discussion
3.1. Optimization of the Hydrolysis Process
Hydrolysis of α-lactalbumin with Alcalase enzyme preparation on buffer phosphate solution (pH 7, 100 mM) was studied as a strategy for the valorization of whey protein fractions, a by-product usually discarded by small and medium dairy enterprises. Initially, protein content and hydrophobicity were determined in the supernatants of the hydrolysates samples 1 to 7. Sample 3 formed a gel after heating at 100˚C for 10 minutes, probably because of the reaction conditions that favoured α-lactalbumin intermolecular interaction by disulfide bonds. For this reason, sample 3 was discarded for subsequent analysis.
It could be observed that protein content measurements tended to increase with factors time of hydrolysis and r (enzyme to substrate ratio, see Table 1). Conversely, surface hydrophobicity decreased after 60 min of hydrolysis at factor r = 0.1000.
Hydrolysis carried out by subtilisin-like enzymes (such as alcalase) might convert some hydrophobic groups to hydrophilic groups by generating two-end carbonyl and amino groups [42] [43] . Furthermore, enhanced protein hydrolysate content can be attributed to the release of small soluble peptides and new carboxylic and amine groups from aminoacids, increasing exposure of more polar and charged groups to surrounding water [44] , therefore reducing hydrophobicity values.
Hydrolysis percentages determined by SE-HPLC were 0 for samples with 0 minutes of reaction as expected. Samples 5, 6 and 7 have the same reaction conditions and presented no significant differences between them (p < 0.05) as expected. Sample 4 had the higher hydrolysis percentage of all samples related to greater protein hydrolysis.
To continue with the characterization of hydrolysates, electrophoresis gels were made (Figure 1). As it can be observed in Figure 1, samples 1 and 2 (lanes 1 and 2, respectively) show the same bands as α-lactalbumin (lane 8) confirming there was no hydrolysis at 0 minutes of reaction. As to samples 4, 5, 6 and 7, no band can be discriminated probably because of the extent of hydrolysis reaction (Table 1) forming small peptides which could have escaped the gel pore.
![]()
Figure 1. Electrophoresis gel of the α-lactalbumin hydrolysates and the molecular weight marker. Lanes 1 and 2: samples 1 and 2, respectively; lane 3, 5, 6 and 7: samples 4, 5, 6 and 7, respectively; lane 4: molecular weight marker (MWM); lane 8: α-lactalbumin.
According to Peng et al. [45] , hydrolysis percentage of whey protein isolate after 5 hours of enzymatic hydrolysis with Alcalase was of 35% - 36%, which is a little bit higher than sample 4 percentage of hydrolysis. In such study, this percentage increased in the first 3 hours reaching a plateau at 5 hours. The value of 3 hours of hydrolysis would be comparable to the one of sample 4 (1 hour of hydrolysis) which could be explained because of being a mix of whey proteins in contrast with α-lactalbumin isolate, and because of different hydrolysis conditions. Adjonu et al. [3] treated whey protein isolate with chymotrypsin, pepsin and trypsin reaching lower percentage of hydrolysis. Alcalase hydrolysates appear to be more hydrolyzed than digestive enzymes, possibly because of its non-specificity [3] producing a great quantity of short peptides with bioactive properties [9] [46] .
3.2. Bioactive Properties.
3.2.1. Antioxidant Capacity
The antioxidant capacity was determined by ABTS and ORAC-FL methods evaluating the relation between the two factors (enzyme: substrate ratio and time) and the antioxidant capacity. The coefficients obtained from multiple linear analyses are listed in Table 2. Both response variables (ABTS and ORAC-FL) have similar tendencies. The coefficient values show a positive effect on the factor time. The factor enzyme: substrate ratio was discarded because of showing a p-value higher than 0.01. As to the model, in both cases R2 is near 1 so it can be concluded that the variation of the antioxidant capacity with the factors fits the model adequately (p < 0.01).
Figure 2 shows surface plots of the antioxidant capacity as the response variable, measured by ABTS and ORAC-FL methods, as a function of enzyme: substrate ratio (0.0050 - 0.1000 w/w) and time (0 - 60 minutes). Both graphs show similar tendencies increasing the antioxidant capacity with time, showing a maximum with 0.1000 w/w and 60 minutes (sample 4). Sample 4 presented 1.015 ± 0.042 and 1.495 ± 0.114 μmol TE/mg of protein, compared to α-lactalbumin 0.191 ± 0.007 and 0.159 ± 0.011 μmol TE/mg of protein, for ABTS and ORAC-FL antioxidant capacity, respectively. Sample 4 values of antioxidant capacity showed significant differences between the other samples, having grater antioxidant power. In addition, IC50 values were obtained for sample
![]()
Table 2. Coefficients of the Equation (1) and statistics obtained for the response surface model by multiple linear regression analysis, for ABTS and ORAC-FL response variables.
r: enzyme: substrate ratio; t: time; R2: determination coefficient; p: p-value for the unfit of the model (coefficients were p < 0.01).
![]() (a)
(a)![]() (b)
(b)
Figure 2. Surface plot of antioxidant activity as the response variable, determined by (a) ABTS and (b) ORAC-FL methods as a function of time (minutes) and enzyme: substrate ratio (w of enzyme/w of α-lactalbumin).
4 (1.018 ± 0.026 and 0.152 ± 0.010 mg/mL of protein for ABTS and ORAC-FL, respectively) and α-lactalbumin (15.732 ± 0.256 and 0.223 ± 0.014 mg/mL of protein for ABTS and ORAC-FL, respectively). These values confirm sample 4 has a higher antioxidant capacity stating it increases with the percentage of hydrolysis [46] confirmed by SE-HPLC (Section 3.1). This suggests that short peptides liberated during hydrolysis are responsible for the antioxidant capacity” [13] being liberated during hydrolysis. These values are similar to those of other enzymes using longer time of reaction [3] [13] , stablishing Alcalase is more efficient than other enzymes, being possible to obtain powerful antioxidant hydrolysates in less time of reaction.
3.2.2. ACE Inhibition Activity
As to antihypertensive properties measured in vitro, sample 4 presented 30% of ACE inhibition with respect to Captopril (data not shown). These low inhibition percentage could be explained by the fact that hydrolysis was not enough to release shorter peptides which are the responsible ones for ACE inhibitory activity [15] . Our recent work is focused on studying the effect of digestion on sample 4 by in vitro simulation which could demonstrate better ACE-inhibitory activity because of enhancing peptide hydrolysis. Hernández-Ledesma et al. [15] already studied the effect of digestion on infant formula demonstrating hydrolysis can enhance ACE-inhibitory activity. Separation of sample 4 lower molecular weight peptides could also enhance this activity applying ultrafiltration for the concentration of these peptides [9] .
3.2.3. Antiglycant Activity
In contrast, no antiglycant in vitro properties were found in sample 4. Moreover, sample 4 (concentration 1 mg/mL of dry mass) demonstrated to favour protein glycation (AGEs formation) in time leveling with positive control for MGO (5 and 10 mM)-BSA and Glc-BSA models (data not shown). AG concentrations of 4 and 8 mM showed complete glycation inhibition (AGEs formation inhibition) for both concentrations of MGO and for Glc during the time of the study. For AG concentration of 1 mM, inhibition of AGEs formation was complete only for Glc model and for MGO was complete until day 6. These results infer MGO is more reactive with sample 4 than Glc, being in accordance with previous researches involving plasma [47] . MGO is more reactive than Glc because of being a dicarbonyl (Maillard reaction intermediate) [21] [48] .
Regarding all the results, sample 4 could be labeled as a powerful antioxidant ingredient for the prevention of chronic diseases related to oxidative stress.
4. Conclusion
The effect of different enzyme: substrate ratio and time conditions on antioxidant activity of α-lactalbumin hydrolyzed with Alcalase was evaluated using response surface methodology, finding greater antioxidant activity in the hydrolysate with 0.1% w/w for enzyme: substrate ratio and 60 minutes for time of reaction (sample 4). The methodology demonstrated time had a positive influence over antioxidant activity because of more hydrolysis (release of shorter peptides), confirmed by SE-HPLC. A pool of short peptides in sample 4 could be responsible for these high antioxidant activities. In contrast, low ACE-inhibitory activity was found in sample 4 probably because of not enough hydrolysis in order to release the tripeptides responsible for these activities, but promising activity could be found after in vivo digestion. As to antiglycant activity, sample 4 showed to favoured AGEs formation with MGO and Glc, not being able to be used as an antidiabetic ingredient.
Acknowledgements
The research that gives rise to the results presented in this publication received funding from the National Agency for Research and Innovation under the code POS_NAC_ 2013_1_11655. Theauthorswouldlike to acknowledge Paula Aphalo and María Cristina Añón of the Centro de Investigación y Desarrollo en Criotecnología de Alimentos (CIDCA) (CCT La Plata, CONICET), Facultad de Ciencias Exactas, Universidad Nacional de La Plata (UNLP), 47 y 116 (1900), La Plata, Argentina for their technical assistance and Laboratorio de Fisicoquímica Biológica (Instituto Química Biológica, Facultad de Ciencias, UdelaR) forthe loan of equipment.