Vanadium Oxide/Graphene Nanoplatelet as a Cathode Material for Mg-Ion Battery ()
1. Introduction
Costly Cheveral phase Mo6S8 cathode, and complicated electrolyte based Mg [ALCL2BuET]2/tetrahydofurane (volatile) are traditional materials in early rechargeable magnesium batteries, although, these batteries suffer from low voltage, low energy density, kinetic sluggish of Mg+2 insertion/extraction [1] [2] [3] [4] . Various cathode materials for magnesium battery have been suggested in recent years, such as TiS2 nanotubes [5] , MoS2 [6] , GeO2 [7] , TiS3 [1] , V2O5 [8] and MnO2 [9] . Vanadium pentaoxide (V2O5) is a semiconductor material (Eg = 2.4 eV), where tuning of fermi level due to ion insertion is expected. The intercalation reaction of Mg in V2O5 can be written as follows: 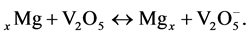 During this reaction the vanadium atoms are partially reduced from a V5+ to a V+4 formal oxidation state [10] . V2O5 is belonging to the layered transition metal oxides which possess the ability to structural deformations during the insertion of Mg+2 or other bivalent ions. The superior electrochemical performances of V2O5 could be ascribed to the unique structure revealing the presence of a large (001) crystal planes interlayer spacing (~11.53 Å), which provide large interlayer spacing for facile ion insertion/extraction [11] [12] [13] . V2O5 has been studied very intensively as a cathode material for Li-ion batteries [14] . Because the ionic radii of Li+ and Mg2+ are comparable in magnitude, 68 and 65 pm, respectively, the replacement of Li+ ions by Mg2+ ions in insertion compounds is possible. On the other hand, although the electrochemical performance of V2O5 has shown great improvement, it suffers from its poor electronic conductivity, which may lead to both of poor capacity and the cyclic ability of V2O5 electrodes. Graphene nanoplatelets represent a new class of carbon nanoparticles with multifunctional properties. Graphene nanoplatelet addition can provide barrier properties, while their pure graphitic composition makes them excellent electrical [15] and thermal conductors and can prevent the vanadium dissolution, and alleviate the aggregation of the particles. Since graphene, the name given to a flat monolayer of carbon atoms tightly packed into a two-dimensional (2D) honeycomb lattice, exhibits superior electrical conductivities, high surface areas and chemical tolerance intrigue, many researchers have studied the V2O5/graphene cathode [16] using different preparation methods to control particle size and particle shape, aiming to improve the efficiency of rechargeable batteries. In the present work we will introduce a new rechargeable magnesium battery from electrolyte system based on reaction products of MgNO3∙6H2O, succinonitril, tetraethylene glycol dimethyl ether solvent, and ((V2O5)0.75/GNP0.25) nanocomposite cathode. One of our important goals is to reduce the cost of the battery. So the starting materials will be from market and the ball mill process is the desirable one.
During this reaction the vanadium atoms are partially reduced from a V5+ to a V+4 formal oxidation state [10] . V2O5 is belonging to the layered transition metal oxides which possess the ability to structural deformations during the insertion of Mg+2 or other bivalent ions. The superior electrochemical performances of V2O5 could be ascribed to the unique structure revealing the presence of a large (001) crystal planes interlayer spacing (~11.53 Å), which provide large interlayer spacing for facile ion insertion/extraction [11] [12] [13] . V2O5 has been studied very intensively as a cathode material for Li-ion batteries [14] . Because the ionic radii of Li+ and Mg2+ are comparable in magnitude, 68 and 65 pm, respectively, the replacement of Li+ ions by Mg2+ ions in insertion compounds is possible. On the other hand, although the electrochemical performance of V2O5 has shown great improvement, it suffers from its poor electronic conductivity, which may lead to both of poor capacity and the cyclic ability of V2O5 electrodes. Graphene nanoplatelets represent a new class of carbon nanoparticles with multifunctional properties. Graphene nanoplatelet addition can provide barrier properties, while their pure graphitic composition makes them excellent electrical [15] and thermal conductors and can prevent the vanadium dissolution, and alleviate the aggregation of the particles. Since graphene, the name given to a flat monolayer of carbon atoms tightly packed into a two-dimensional (2D) honeycomb lattice, exhibits superior electrical conductivities, high surface areas and chemical tolerance intrigue, many researchers have studied the V2O5/graphene cathode [16] using different preparation methods to control particle size and particle shape, aiming to improve the efficiency of rechargeable batteries. In the present work we will introduce a new rechargeable magnesium battery from electrolyte system based on reaction products of MgNO3∙6H2O, succinonitril, tetraethylene glycol dimethyl ether solvent, and ((V2O5)0.75/GNP0.25) nanocomposite cathode. One of our important goals is to reduce the cost of the battery. So the starting materials will be from market and the ball mill process is the desirable one.
2. Experimental
Graphene Nanoplatelets Grade M GNP was characterized by average (7 nm thickness, 10 nm particle diameter, 107 S/m Electrical conductivity and surface area ~120 - 150 m2/g) have been imported from XG Science company. Composites of V2O5 and GNP were prepared by ball milling process under 4 hours’ time duration. The resulted product is designated here as (V2O5)1−x(GNP)x composites. The morphology of the nanocompsite was examined using SEM (JOEL-JSM Model 5600). The XRD patterns of the films were taken using Rigaku diffractometer type RINT-Ultima IV/S. The diffraction system based with Cu tube anode with voltage 40 KV and current 40 mA. The current?voltage characteristics of the cathodes were carried out by means of a computer controlled 2400 Keithley electrometer. For electrochemical performance testing, working electrodes (V2O5 and V2O5-graphene composite powder) were prepared by mixing 85 wt.% sample as the active material, 6 wt.% conductive agent (carbon black, Super-P-Li), and 9 wt.% poly-vinylidene difluoride (PVDF) binder, N-methylpyrrolidone (Alfa) was then added to produce a viscous slurry and the resultant slurry was pasted onto copper foil. The as-prepared working electrodes were then dried in a vacuum oven at 373 K for 2 h. Electrochemical cells (CR2032 coin type) were assembled in room temperature and ambient pressure by using the working electrode, a separator (filter paper), Mg ribbon as the reference and counter electrode, and 4 gm MgNO3∙6H2O in a 2:10 (w:v) mixture of succinonitril, tetraethylene glycol dimethyl ether, respectively, as the electrolyte. Figure 1 shows the structure scheme of MgNO3.6H2O, succinonitril and tetraethylene glycol dimethyl ether. Where MgNO3.6H2O is the Mg+2 pump, tetraethylene glycol dimethyl ether is a solvent and succinonitril is a plasticizer agent to dissociate ions and improve ionic conductivity. Cyclic voltammograms (CVs) were conducted in three-electrode cell using an electrochemical instrument of CHI604E Electrochemical Workstation. The cells were charged and discharged on a multi-channel battery test system (NEWARE BTS-TC35) over the voltage range of 0 - 1.6 V versus Mg/Mg+2 at constant constant current density ~40 μAcm−1.
3. Results and Discussion
Figures 2(a)-(c) shows SEM images for V2O5 and V2O5/GNP nanocomposites. The graphene nanoplatlets are crumpled to a curly and wavy shape. By adding GNP nanoplatlets to V2O5, the later was found to be uniformly distributed in GNP and well distributed on the 2D graphene nanoplatelets, as shown in Figures 2(a)-(c). Moreover, the graphene sheets can prevent the aggregation of V2O5 particles to a certain extent, which can be of great benefit to electrochemical reactions. The XRD patterns of the synthesized (V2O5)1−x(GNP)x composites (where x = 0, 5, 10, 15, 20 and 25 %wt GNP) are shown in Figure 3. The XRD spectrum for x = 0% indicates that the V2O5 composites
![]()
Figure 1. Schematic illustrates the electrolyte structure.
![]()
Figure 2. SEM micrographs of (V2O5)1−x(GNP)x composites: (a) 0 wt% GNP; (b) 15 wt% GNP; (c) 25 wt% GNP.
![]()
Figure 3. XRD pattern of (V2O5)1−x(GNP)x composites.
are highly crystallized in structure and the entire diffraction peaks match well with Bragg reflections of the pure orthorhombic phase of V2O5 nanoparticles, which is consistent with the standard JCPDS No.41-1426 (space group Pmmn) [17] [18] . However, for x > 0% an additional diffraction shoulder peak around 26.77˚, partially overlapping with the V2O5 (110) peak (2θ = 26.39˚), originates from the (002) diffraction of the graphite. The intensity of the later peak increases linearly when x increases from 5 to 25% and its position corresponds to ~ 31 nm (according to the relation: λ/(2∙sinθ, θ = 26.39˚) spacing between atomic planes [19] . Also, the XRD spectra show a weak peak at 54.8˚ which corresponds to the (004) reflection of the graphite. It can be noticed that the graphite (002) peak position shifts to the lower angles for x = 5% and to higher angles for x = 10%. This can be related to the amount of oxygen functional groups formed between the platelets of the graphite. From the XRD measurements, no additional phases related to structural defects can be detected, while orthorhombic V2O5 can be detected after ball milling of V2O5 and GNP. Small shift in peak positions of V2O5 after doping by GNP was observed which confirm change in (001) interlayer spacing. The crystallite size (τ) of the investigated samples (V2O5)1−x(GNP)x nano-composites (where x = 0, 5, 10, 15, 20 and 25 %wt GNP) can be calculated using the first sphere approximation of Debye-Scherrer formula [20] τ = K l/βcosθ, where K is the shape factor, λ is the X-ray wavelength, β is the line broadening at half the maximum intensity in radians, and θ is the Bragg angle. Using this formula, crystallite dimensions of about 30 nm could be calculated from the high intense peaks. Figure 4(a), shows the current voltage (I&V) characteristics of (V2O5)1−x(GNP)x nano-composite. Generally, the current increases linearly with increasing voltage obeying Ohm law. The dc conductivity
was calculated using the equation: 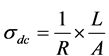 [21] , where t is the thickness of the
[21] , where t is the thickness of the
sample and A is the surface area of the sample. The value of the resistivity R was measured from the slopes of the straight lines in ohmic region I α V Figure 4(a), The effect of graphene content on the dc conductivity σdc of (V2O5)1−x(GNP)x is shown in Figure 4(b). The dc conductivity of the composites exhibits insulator behavior for pure V2O5 recording ~10 × 10−6 S/m, whereas semiconductor behavior for low graphene content up to x = 10 wt% was recorded. The electrical conductivity increased as the content of graphene was close to percolation threshold up to x = 5 wt%. Above the percolation threshold σdc was found to increases exponentially before it reach to the saturated point (x = 10 wt%) which may be attributed to the formation of filler network and the composites may reach to the metallic behavior. This behavior can be described according to the percolation theory as the following power relation [22] ,
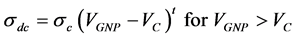
where 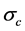 is the conductivity of conducting component,
is the conductivity of conducting component, 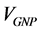 is the volume fraction of Graphene,
is the volume fraction of Graphene, 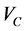 is the critical volume fraction or percolation threshold, and the exponent t reflects the dimensionality of the system and has been calculated to be either 1.3 or 2.0 corresponding to two or three dimensions, respectively [23] . Figure 4(b) (inset) shows a fitting of percolation equation for (V2O5)1−x(GNP)x composites. The exponent t was found to be about 1.4. This result confirms that the GNP nanoparticles are not located on the surface of the host material matrix particles, but it coordinated in the V2O5 crystal structure and the formation of graphene three dimensional network will enhancement.
is the critical volume fraction or percolation threshold, and the exponent t reflects the dimensionality of the system and has been calculated to be either 1.3 or 2.0 corresponding to two or three dimensions, respectively [23] . Figure 4(b) (inset) shows a fitting of percolation equation for (V2O5)1−x(GNP)x composites. The exponent t was found to be about 1.4. This result confirms that the GNP nanoparticles are not located on the surface of the host material matrix particles, but it coordinated in the V2O5 crystal structure and the formation of graphene three dimensional network will enhancement.
Cyclic voltammetry and discharge curve
Figure 5(a) shows the schematic of the cell configuration of the Mg− V2O5 cells in this study. The activity of V2O5 and V2O5-graphene nanocomposite for hosting Mg+2 ions was evaluated using cyclic voltammetry (CV) and galvanostatic discharge-charge techniques. Figure 5(b), shows the CV results obtained from 2.5 to 0 V using V2O5 and V2O5-graphene nanocomposite as a working electrode in a three-electrode cell employing magnesium metal as the counter and reference electrode at a scan rate of 0.05 mV s−1. Although GNP succeeded to increase conductivity of V2O5, it failed to increase
![]()
Figure 4. (a) I-V curves of (V2O5)x/(GNP)1−x composites; (b) Variation of dc conductivity of (V2O5)x/(GNP)1−x composites at different grapheme concentrations
![]()
Figure 5. (a) Schematic illustrates Mg+2 insertion/extraction within V2O5; (b) Comparison of the CV curves (at 5 mV∙s−1) for V2O5 and V2O5/graphene nano-composite.
![]()
Figure 6. Discharge-charge profiles of (a) V2O5 (b) V2O5/graphene nano-composite cathodic materials.
the cathodic and anodic current density. This can be attributed to that the introduction of GNP in V2O5 using ball mill technique can perturb the (001) interlayer spacing of V2O5 and may be reduce intercalation rate. The perturbation in the interlayer spacing probably decreases the probability of Mg+2 insertion/extraction between interlayer spacing and hence decrease the specific capacity as we will see.
Figure 6(a) & Figure 6(b) show discharge/charge profiles of Mg/V2O5 and Mg/(V2O5/GNP) coin cells, in which current density was fixed at =40 μAcm−1 and the cells were discharged to 0 V and charged to 1.6 V. The initial discharge capacity of Mg/ V2O5 and Mg/(V2O5/GNP) coin cells are approximately 100 and 90 mAhg−1, respectively. The combination of V2O5 with GNP decrease the discharge capacity compared to pure V2O5. We could not obtain more than 2 and 4 cycles for pure and graphitized V2O5, respectively. We think the structure property of V2O5 which give the advantage (001 large interlayer spacing (∼11.53 Å)) of facile Mg+ insertion/extraction was deformed after initial cycling. The intercalation of Mg+2 may perturb the bonding scheme of V2O5 and losses it this property.
4. Conclusion
In summary, V2O5/GNP cathode was synthesized by a ball mill method. The integration of V2O5 and graphene nanoparticles enhanced the electrical performances. This improved performance could be attributed to the formation of framework nanoscale electrode of 2D graphene decorated with well-dispersed V2O5 nanoparticle. Although GNP perturbed the (001) interlayer spacing hence, it failed to enhance the electrochemical performance. As-prepared V2O5 and V2O5/GNP cathodes can deliver a high capacity of 100 and 90 mAh∙g−1 respectively, which provides a new direction to explore cathode materials for rechargeable Mg batteries. Further extensive investigations are required, however, to raise the performance of the magnesium-based rechargeable cells to practical levels.