Synthesis and Investigation of Phenol Red Dye Doped Polymer Films ()
Received 1 March 2016; accepted 21 May 2016; published 24 May 2016

1. Introduction
The interest in the optical properties of organic materials has grown due to the wide range of applications in photonic devices, such as sensors, light emitting diodes, solar cells, limiters, and optoelectronic devices [1] - [10] . The data obtained from the spectra of absorbance (A), transmittance (T), and reflectance (R) of these materials can be used to determine their intrinsic parameters. These include, absorption coefficient (α), extinction coefficient (κ), refractive index (n), optical and electrical conductivities (σopt and σelect), and optical energy band gap (Eg). Investigation of these parameters is considerably important for the development of materials that can be used as components in the optoelectronic devices.
Organic dyes provide promising materials for photonic devices due to their excellent optical properties [10] - [14] . Phenol Red dye is one of these materials. As we find from the present study, this material has several good properties such as, high fabrication flexibility, good photo-chemical stability, high refractive index, and high dielectric constant. Poly (methyl-methacrylate) (PMMA) polymer is an important and interesting polymer can be used as host medium because of the attractive physical and optical properties decisive about its broad applications. This material has some advantages such as, good transparency in the visible region of the electromagnetic spectrum, thermal stability, and relatively high damage resistance for the laser beams [4] [15] . Introducing the Phenol red dye (as a dopant) in the PMMA will cause significant changes in their physical properties. There are various techniques, which are used to prepare solid polymer films [16] [17] . Among these techniques, casting technique is a simple and effective technique to obtain polymer films with good optical quality, as well as; it has a relatively low manufacturing cost.
In the present work, pure and Phenol Red dye doped polymer films were prepared by casting method. The optical properties of the prepared films were investigated and their useful optical parameters were determined. The effect of dye concentration on the values of these parameters was also studied.
2. Materials and Experimental
The molecular formula of the Phenol Red dye is C19H14O5S, and its chemical structure is shown in Figure 1. The pure and dye doped polymer films were prepared using the casting technique. Appropriate amount of poly (methyl-methacrylate) (PMMA) polymer was dissolved in a mixture of chloroform solvent and 5% of the volume of methanol, as additive solvent. The mixture of the two solvents is suitable for both the dye and polymer. Product was stirred using a magnetic stirrer until the polymer is completely dissolved and homogeneous solution produced. Then suitable quantities of the mixture (2 ml for each sample) were cast on thin glass slides and kept until the solvent gradually evaporated at room temperature and solid films obtained. The dye doped polymer films were prepared as follows: A Phenol Red dye solution with certain concentration was prepared by dissolving appropriate amount of dye powder in a mixture of chloroform and 5% of the volume of methanol. The produced solution was diluted by different ratios of the solvent in order to obtain solutions with different concentrations: 0.05, 0.30, 0.40 mM. Then, required amounts of PMMA polymer were added to each dye solution and the procedures for preparation of the pure polymer films were repeated. The obtained films were examined and found that they are exhibiting good optical transparency in the visible region. The thickness of the obtained films was in the range 0.85 - 1 mm. For our measurements, the films with thickness 1 mm were used.
The Ultraviolet-Visible (UV-Vis) absorption spectra of prepared Phenol Red dye doped polymer films were characterized by using Cecil double-beam spectrophotometer (model CE-75000) with the wavelength range 190 - 1100 nm. An uncoated glass slide was used as a reference sample for the absorption measurement.
![]()
Figure 1. Chemical structure and molecular formula of Phenol Red dye.
3. Results and Discussion
The spectral distributions of the absorbance (A), the transmittance (T), and the reflectance (R) of the Phenol Red dye doped polymer films at different concentrations were plotted as a function of the wavelength in Figures 2-4, respectively. In Figure 2, the absorbance spectra of dye doped polymer films at the concentrations 0.30 mM and 0.40 mM exhibited broad band centered at 418 nm, while at the low dye concentration 0.05 mM, the absorbance spectrum does not show clear absorption peak. The transmittance reached to 80% - 90% for the Phenol Red dye doped polymer films at the low dye concentration 0.05 mM and it decreased with increased the dye concentration, as shown in Figure 3 for the dye concentrations 0.30 mM and 0.40 mM. It is seen from Figure 4 that the spectrum curves of the reflectance of the Phenol Red dye doped polymer films show behaviors similar to those of the absorbance; this is attributed to the correlation between reflectance and the absorbance. The value of R at the low dye concentration 0.05 mM is only 5% in the visible wavelengths region and increases with increasing the dye concentration. This value reached to 21% at the dye concentration 0.40 mM; at wavelength 418 nm. The absorbance (A) of light by an optical medium is quantified by its absorption coefficient (α). This is defined by the Beer-Lambert’s law [18] [19] :
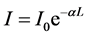 (1)
(1)
![]()
Figure 2. UV-Vis absorbance spectra of Phenol Red dye doped polymer film at different concentrations.
![]()
Figure 3. UV-Vis transmittance spectra of Phenol Red dye doped polymer film at different concentrations.
where I0 is the incident intensity of light, I is the output intensity of light, and L is the thickness of the absorbing medium. Equation (1) can be written as follows:
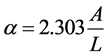 (2)
(2)
where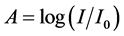 .
.
The values of α of the Phenol Red dye doped polymer films were calculated using Equation (2). The variation of α with the photon energy (hν) at different concentrations is displayed in Figure 5. It is seen that the value of α increases with increasing the photon energy (hν) until reached to its maximum value at incident photon energy hν ≈ 2.97 eV and then starts to decrease with increasing hν. This is clearly evident with high dye concentrations. It is also seen that increasing dye concentration leads to significant increase in the value of α. For the dye concentration range (0.05 - 0.40) mM, the values of α vary within the range (1.0 - 8.5) cm−1.
Extinction coefficient (k) of material is related to the absorption coefficient (α) according the following relation [20] :
![]()
Figure 4. The reflectance (R) of the Phenol Red dye doped polymer film versus the incident photon energy (hν), for different dye concentrations.
![]()
Figure 5. The absorption coefficient (α) of the Phenol Red dye doped polymer film versus the incident photon energy (hν), for different dye concentrations.
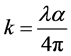 (3)
(3)
where λ is the wavelength of the incident photon.
Using the values of the corresponding α and Equation (3), the values of k for the Phenol Red dye doped polymer films at different concentrations were calculated for different values of incident photon energy (hν). The variation of k of the Phenol Red dye doped polymer film with the photon energy (hν) is plotted in Figure 6 for different dye concentrations. It is noticed that the variation of k with hν has behavior similar to that of α because of the direct relation between the two constants through the relation (3). The values of k vary within the range (7 - 29) × 10−6 for the concentration range (0.05 - 0.40) mM.
The refractive index (n) of the Phenol red polymer film was calculated using the relation [20] [21] :
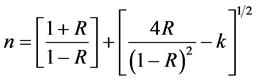 (4)
(4)
where the optical reflectance (R) is given by the relation [16] [20] [21] :
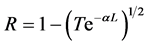 (5)
(5)
Figure 7 shows the variation of the refractive index (n) of the Phenol Red dye doped polymer film with incident photon energy (hν) for different dye concentrations. The values of n are within the range (1.40 - 2.50) for the concentration range (0.05 - 0.40) mM.
The optical conductivity of the material is given by the following relation [20] :
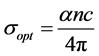 (6)
(6)
where c is the velocity of light.
The electrical conductivity (σelect) of the Phenol Red dye doped polymer film is related to its optical conductivity (σopt) according to the following relation [20] :
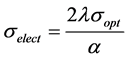 (7)
(7)
We used, respectively, Equations (6) and (7) to calculate σopt and σelect of the Phenol Red dye doped polymer films. The variations of σopt and σelect with incident photon energy are shown in Figure 8 and Figure 9, respectively, for different dye concentrations. The evaluated values of σopt are within the range (0.40 - 5.00) × 1010
![]()
Figure 6. The extinction coefficient (κ) of the Phenol Red dye doped polymer film versus the incident photon energy (hν), for different dye concentrations.
![]()
Figure 7. The refractive index (n) of the Phenol Red dye doped polymer film versus the incident photon energy (hν), for different dye concentrations.
![]()
Figure 8. The variation of the optical conductivity (σopt) with the incident photon energy (hν) for the Phenol Red dye doped polymer films at concentrations 0.05, 0.30, and 0.40 mM.
![]()
Figure 9. The variation of the electrical conductivity (σelect) with the incident photon energy (hν) for the Phenol Red dye doped polymer films at concentrations 0.05, 0.30, and 0.40 mM.
(sec)−1, while the values of σelect are within the range (3 - 6) (Ω∙cm) −1, over the dye concentration range (0.05 - 0.40) mM. It is seen that the value of σelect is high at the low photon energies and decreases with increasing hν.
The optical energy band gap (Eg) of the film is related to its absorption coefficient (α) and the energy of the incident photon (hν) according to the following relation [18] [21] [22] :
![]() (8)
(8)
where h is the Planck’s constant, ν is the frequency of incident radiation, B and m are constants. The value of m is dependent on the type of transition between the valence band and the conduction band of the material. m takes the values: 2, 3, 1/2, or 3/2, for allowed direct, forbidden direct, allowed indirect, or forbidden indirect, transitions, respectively. In the present work, the analysis of UV-V is absorption spectra revealed that the dye doped polymer films, have allowed indirect transitions, therefore the value of m = 1/2 was used in Equation (8) for the Eg determination. The values of Eg of the pure and the Phenol Red dye doped polymer films, with the dye concentrations 0.05, 0.30, and 0.40 mM, were obtained from extrapolating the straight-line portions of the curves (αhν)1/2 versus hν to the hν-axis (where αhν = 0), as shown in Figure 10. For clarity, the three graphs were plotted separately in Figure 10. The obtained values of Eg are summarized in Table 1. The Eg value of the pure PMMA polymer film was determined to be 4.73 eV. This value is comparable to the value reported by other workers [23] [24] . It was observed that the addition of Phenol Red dye at different concentrations in PMMA polymer changed the value of Eg. The value of Eg decreased with increased the doping concentration. The values
![]()
Table 1. The obtained values of optical band gap energy (Eg) for the pure PMMA polymer film and the Phenol Red dye doped polymer films at different concentrations.
of Eg for the dye doped polymer films were found to be in the range of (4.71 - 1.61) eV, when the dye concentration increased over the range (0.05 - 0.40) mM. The decrease in the value of Eg might be due to the formation of defects in the polymer matrix, which results in creation of localized sublevels in the optical band gap and consequently decreased the energy band gap.
4. Conclusion
The pure and Phenol Red dye doped polymer films were synthesized using the casting technique and their optical properties were investigated for different dye concentrations. The main optical parameters of the dye doped polymer films were determined for different dye concentrations. Results showed that the optical properties of the PMMA polymer film were appreciably modified in the presence of the organic Phenol Red dye. The optical energy band gap (Eg) values of the prepared polymer films show the decreasing trend with an increasing concentration of Phenol red dye. The obtained results from the investigation suggest that the prepared Phenol Red dye doped polymer films are useful for potential applications in solar cells, as well as in optoelectronic and photonic devices.
NOTES
![]()
*Corresponding author.