Utility of Styrylpyrazoloformimidate in the Synthesis of Fused Heterocyclic Compounds ()
1. Introduction
The chemistry of hydrazonoyl halides has attracted the interest of many research groups as they have proved to be useful organic synthesis [1] - [10] . In continuation of our long standing interest for the utility of nitrilimines derived from hydrazonoyl halides in the synthesis of heterocycles [11] - [14] , we are interested in (Z)-N'-phenyl- cinnamohydrazonoyl chloride 1 to study the effect of C=C double bond on the cycloaddition reactions [15] - [17] . We wish to report herein a simple and convenient route for the synthesis of pyrazolo[3,4-d]pyrimidine, pyrazolo[4,3-e][1,2,4]triazolo[4,3-c]pyrimidines and its isomeric pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidine derivative via Dimroth rearrangement. Such compounds have been used as a new pharmacological test for characterization of human A3 adenosine receptors [18] - [20] .
2. Results and Discussion
Compound (E)-5-amino-1-phenyl-3-styryl-1H-pyrazole-4-carbonitrile 2 was prepared from our laboratory via reaction of (Z)-N'-phenylcinnamohydrazonoyl chloride 1 with malononitrile in ethanolic sodium ethoxide solution (Scheme 1) [21] . Refluxing of compound 2 with triethylorthoformate in acetic anhydride afforded ethyl N-(4-cyano-1-phenyl-3-((E)-styryl)-1H-pyrazol-5-yl)formimidate 3 (Scheme 1). The structure of compound 3 was established on the basis of elemental analysis and spectral data. The IR spectrum of 3 revealed the absence of amino group, while it showed a characteristic band at υ 2215 cm−1 assignable to cyano group. Its 1H NMR data showed signals at δ, 1.29 (t, 3H, CH3), 4.32 (q, 2H, CH2), 7.15 - 7.69 (m, 12H, Ar H), and 8.60 (s, 1H, NCH). Also, its 13C-NMR spectrum showed 17 carbon atoms. Moreover, the mass spectrum showed molecular ion peak as a base peak at m/z 342 (100%). Reaction of 3 with hydrazine hydrate in ethanol at room temperature yielded a product 4 which analyzed correctly for C19H16N6 (Scheme 1). The IR spectrum of 4 showed the absence of cyano group and it showed bands at υ 3351, 3309, 3177 cm−1 assignable to amino and imino groups. Also, the mass spectrum revealed a base peak at m/z 328 (100%) corresponding to its molecular ion peak. On the basis of elemental analysis and spectral data, the product is (E)-4-imino-1-phenyl-3-styryl-1H-pyrazolo [3,4-d]pyrimidin-5(4H)-amine 4.
When compound 4 was refluxed with diethyl dicarbonate a single product was obtained, its mass spectrum and elemental analysis are consistent with the molecular formula C23H18N6O2 (Scheme 2). Two possible structures were proposed for the isolated product 5a and 5b. The identity of the isolated product was confirmed to be (E)-ethyl-7-phenyl-9-styryl-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidine-2-carboxylate 5b. Thus, sapo- nification of the reaction product obtained 5b gave the intermediate acid 6 which decarboxylated to a pro- duct identical to all respects (m.p., mixed m.p., IR) with (E)-7-phenyl-9-styryl-7H-pyrazolo[4,3-e][1,2,4]triazo- lo[1,5-c]pyrimidine 7. The latter product 7 was also confirmed via its alternative synthesis by treatment of compound 4 with triethylorthoformate or formic acid. Structure of 5a was accordingly discarded. In addition, structure 5b was further substantiated by IR and 1H NMR spectra. Its IR spectrum exhibits a carbonyl band at υ 1743 cm−1 and 1H NMR spectrum showed signals at: δ 1.42 (t, J = 7 Hz, 3H), 4.49 (q, J = 7 Hz, 2H), 7.37-8.78 (m, J = 7 Hz, 12H), and 9.84 (s, 1H, pyrimidine-CH).

Scheme 1. Synthesis of formimidate 3 and amino imino compound 4.
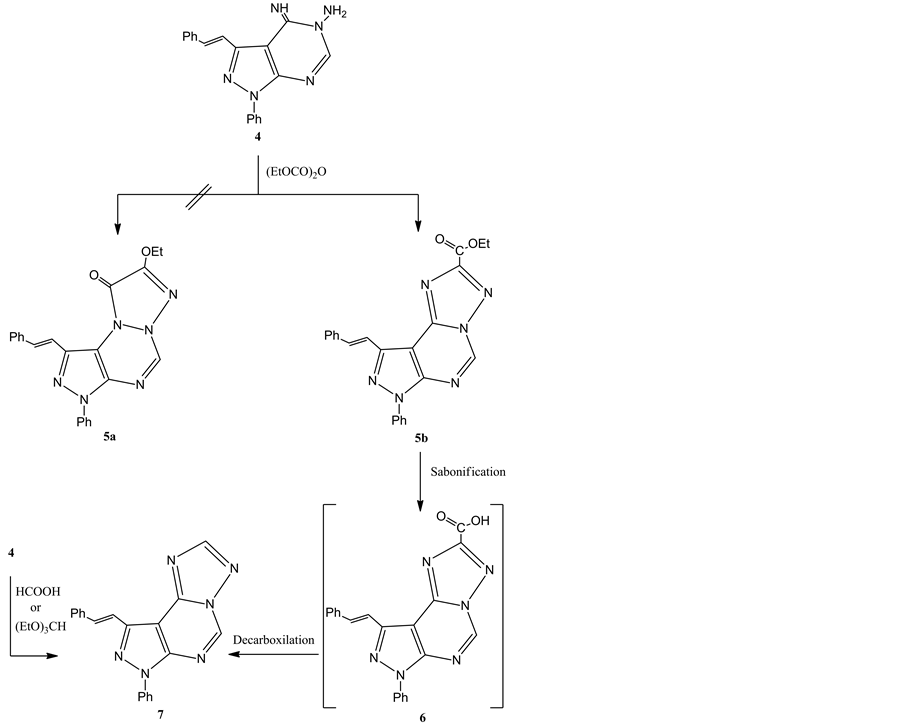
Scheme 2. Synthesis of pyrazolotriazolopyrimidine 7.
Refluxing of compound 4 with hydrazine hydrate in ethanol, gave (E)-4-hydrazinyl-1-phenyl-3-styryl-1H- pyrazolo[3,4-d]pyrimidine 8, via Dimroth type rearrangement, which has not been reported hitherto (Scheme 3 and Scheme 4). This is consistent with a similar rearrangement that was reported recently [22] . The structure of 8 was confirmed by elemental and spectral data (see experimental) and its reaction described below.
Thus, treatment of hydrazine derivative 8 with the appropriate aldehydes 9a-i in refluxing ethanol in the presence of acetic acid led to the formation of the new condensation products, 4-(2-arylhydrazinyl)-1-phenyl -3-styryl-1H-pyrazolo[3,4-d]pyrimidine 10a-i. The structures of 10a-i were confirmed by their elemental analysis and spectral data. For example their IR spectra showed the characteristic band for NH at υ 3199 - 3352 cm−1. Also, their 1H NMR spectra revealed in each case, a signal in the region 11.97 - 12.13 assignable to NH proton which disappeared upon shaking its DMSO solution with D2O.
Oxidative cyclization of hydrazone 10a-h led to the formation of pyrazolo[4,3-e][1,2,4]triazolo[4,3-c]pyri- midine derivatives 11a-h (Scheme 3 and Scheme 5). Thus, stirring of 10a-h with 4 equivalent of Fe(ΙΙΙ) chloride in ethanol overnight gave, in each case, a single product as evidenced by TLC analysis. Mass spectra revealed that, each product has 2 hydrogen atoms less than that of the respective hydrazone. Also, IR and 1H NMR revealed the absence of NH band and CH=N proton, respectively.
Compounds 11a,e were isomerized to the thermodynamically more stable pyrazolo[4,3-e][1,2,4]triazo- lo[1,5-c]pyrimidine derivatives 13a,e through tandem ring opening and ring closure reactions via heating of 11a,e in ethanol in the presence of sodium acetate (Scheme 3). This rearrangement is consistent with those reported in earlier reports [23] . The structures of 13a,e were established by elemental and spectral analysis (see experimental). Also, the structures of 13a,e were confirmed via their alternative synthesis. Thus, treatment of 4 with acid chlorides 12a,e in refluxing pyridine gave a products identical in all respects (m.p., mixed m.p., IR and 1H NMR spectra) with those obtained above from base-catalyzed rearrangement of 11a,e (Scheme 3). Also,
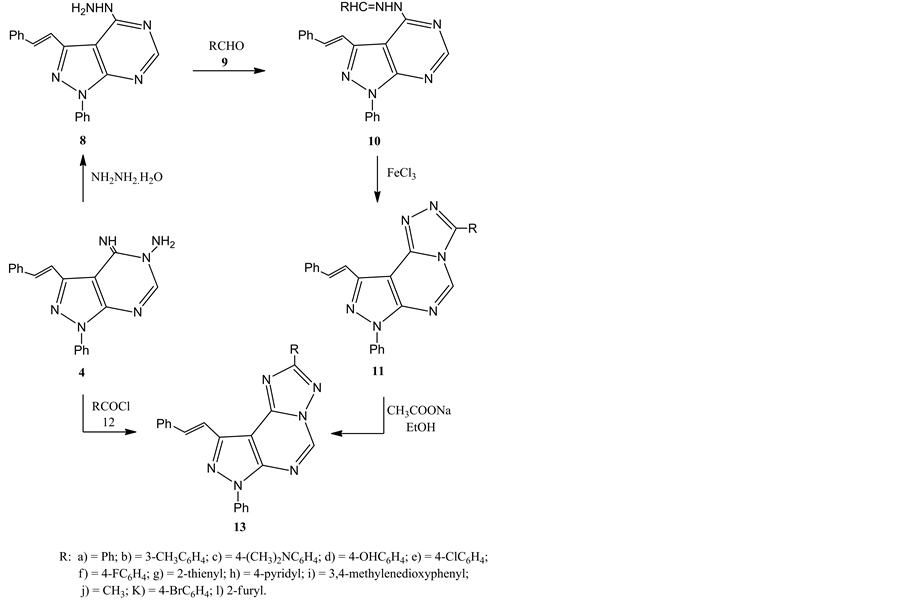
Scheme 3. Synthesis of hydrazinylpyrazolo pyrimidine 8, arylhydrazinylpyrazolopyrimidine 10 and pyrazolotriazolopyrimidine derivatives 11, 13.
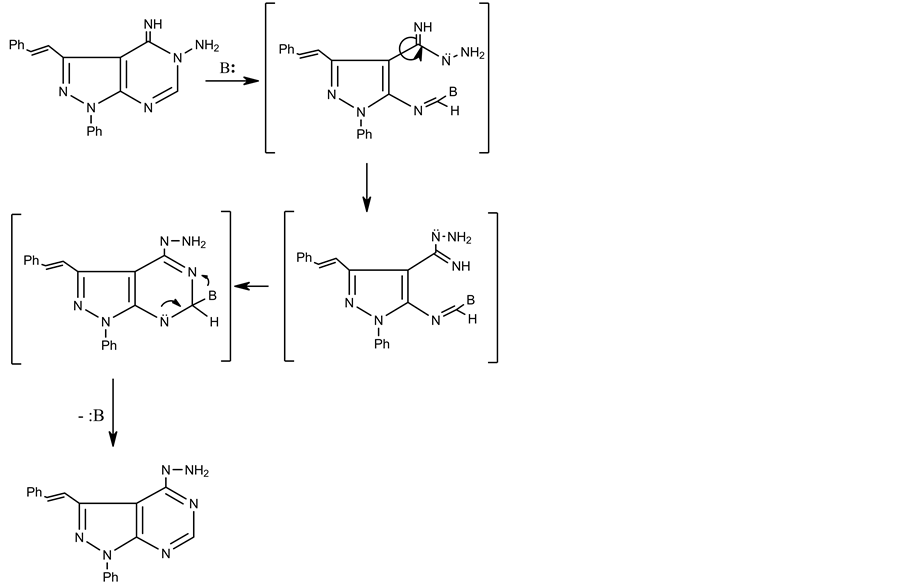
Scheme 4. Synthesis of (E)-4-hydrazinyl-1-phenyl-3-styryl-1H-pyrazolo [3,4-d]pyrimidine.

Scheme 5. Synthesis of (E)-2-alkyl-7-phenyl-9-styryl-7H-pyrazolo[4,3-e][1,2,4] triazolo[1,5-c]pyrimidine.
compound 4 reacted with 12j,k,l to give the corresponding 13j,k,l. The latter compounds 13j,k,l were confirmed by elemental and spectral analysis.
3. Experimental
3.1. General
All melting points were determined on an electrothermal GallenKamp melting point apparatus and are uncorrected. The IR spectra were recorded as KBr Pellets on a Jasco FTIR-460 plus Fourier transform infrared spectrophotometer. 1H and 13C NMR spectra were recorded at (300 MHz) and (75 MHz) respectively on Varian EM-300 MHz spectrometer. Chemical shifts (δ) are given from TMS (ppm) as internal standard for 1H NMR and 13C NMR. Mass spectra were recorded on AEI MS 30 mass spectrometer operating at 70 eV. The elemental analyses were performed at the Microanalytical Center of Cairo University. Compound 2 was prepared as previously described [18] .
3.2. Preparation of Ethyl N-(4-Cyano-1-Phenyl-3-((E)-Styryl)-1H-Pyrazol-5-yl)Formimidate 3
To a solution of the compound (E)-5-amino-1-phenyl-3-styryl-1H-pyrazole-4-carbonitrile 2 (1.43 g, 5 mmol) in acetic anhydride (5 mL), triethylorthoformate (0.74 g, 5 mmol) was added. The reaction mixture was refluxed for 5 h and the solvent was evaporated under reduced pressure. The solid product was collected and crystallized from acetonitrile to afford the compound 3. Yellow crystals; m.p: 170˚C - 171˚C; yield (82%); IR (KBr): υ = 2215 (CN) cm−1; 1H NMR (DMSO-d6): δ = 1.29 (t, 3H, CH2CH3), 4.32 (q, 2H, CH3CH2), 7.15 - 7.69 (m, 12H, Ar H), 8.60 (s, 1H, NCH); 13C NMR (DMSO-d6): δ = 13.74, 64.07, 79.46, 114.46, 117.78, 123.78, 126.88, 128.00, 128.74, 128.85, 128.97, 132.88, 135.58, 137.46, 149.57, 151.51, 162.46. MS: m/z (%) = 342 (M+, 100), 313 (31), 285 (45), 77 (41). Anal. for C21H18N4O: Calcd. C, 73.67; H, 5.30; N, 16.36. found C, 73.31; H, 5.03; N, 16.11.
3.3. Preparation of (E)-4-Imino-1-Phenyl-3-Styryl-1H-Pyrazolo[3,4-d]Pyrimidin-5(4H)-Amine 4
To a solution of the compound 3 (17.1 g, 50 mmol) in ethanol (250 mL), hydrazine hydrate (2.5 mL, 50 mmol) was added. The reaction mixture was stirred for 5 h at room temperature; the solid product was collected and crystallized from dioxane to afford the compound 4. Yellow crystals; m.p. 200˚C - 202˚C; yield (91%); IR (KBr): υ = 3351 & 3309 (NH2), 3177 (NH) cm−1. MS: m/z (%) = 328 (M+, 100), 313 (29), 77 (30). Anal. for C19H16N6: Calcd. C, 69.50; H, 4.91; N, 25.59. found C, 69.07; H, 4.72; N, 25.39.
3.4. Preparation of (E)-9-Styryl-7H-Pyrazolo[4,3-e][1,2,4]Triazolo[1,5-c]Pyrimidine-2-Carboxylate Ethyl 7-Phenyl 5b
A solution of the compound 4 (1.64 g, 5 mmol) in diethyl dicarbonate (10 mL) was refluxed for 4 h. and then cooled. The solid product was filtered off, dried and finally crystallized from acetic acid to give 5b. Yellow crystals; m.p. 212˚C - 214˚C; yield (88%); IR (KBr): υ = 1743 (CO) cm−1; 1H NMR (DMSO-d6): δ = 1.42 (t, 3H, CH2CH3), 4.49 (q, 2H, CH3CH2), 7.37 - 8.78 (m, 12H, Ar H), 9.84 (s, 1H, NCH). MS: m/z (%) = 410 (M+, 96), 409 (50), 77 (100). Anal. for C23H18N6O2: Calcd. C, 67.31; H, 4.42; N, 20.48. found C, 67.02; H, 4.37; N, 20.25.
3.5. Preparation of (E)-7-Phenyl-9-Styryl-7H-Pyrazolo[4,3-e][1,2,4]Triazolo[1,5-c]Pyrimidine 7
A solution of the compound 4 (1.64 g, 5 mmol) in triethylorthoformate or formic acid (10 mL) was refluxed for 4 h. left to cool and the solid product was filtered, dried and finally crystallized from acetic acid to afford 7. Pale yellow crystals; m.p. 198˚C - 200˚C (acetic acid); yield (86%); 1H NMR (DMSO-d6): δ = 6.98 - 8.56 (m, 12H, Ar H), 9.77 (s, 1H, NCH), 9.79 (s, 1H, NCH). MS: m/z (%) = 338 (M+, 98), 337 (100), 77 (30). Anal. for C20H14N6: Calcd. C, 70.99; H, 4.17; N, 24.84. found C, 70.65; H, 4.12; N, 24.61.
3.6. Preparation of (E)-4-Hydrazinyl-1-Phenyl-3-Styryl-1H-Pyrazolo[3,4-d]Pyrimidine 8
A solution of the compound 4 (16.4 g, 50 mmol) in ethanol (250 mL) and hydrazine hydrate (10 mL) was refluxed for 5 h. The solvent was evaporated; the solid product was collected, dried and finally crystallized from acetonitrile to afford 8. Yellow crystals; m.p. 202˚C - 204˚C; yield (90%); IR (KBr): υ = 3310 (NH), 3351 & 3309 (NH2) cm−1; 1H NMR (DMSO-d6): δ = 4.86 (s, 2H, NH2), 7.31-8.41 (m, 13H, Ar H), 9.25 (s, 1H, NH). MS: m/z (%) = (M+, 38), 313 (30), 297 (80), 77 (100). Anal. for C19H16N6: Calcd. C, 69.50; H, 4.91; N, 25.59. found C, 69.22; H, 4.81; N, 25.33.
3.7. General Method for Preparation (E)-4-(2-Arylhydrazinyl)-1-Phenyl-3-Styryl-1H-Pyrazolo[3,4-d]Pyrimidine 10a-i
To a mixture of compound 8 (1.64 g, 5 mmol) in ethanol (30 mL), the appropriate aldehyde 9a-i (5 mmol) was added. The reaction mixture was refluxed for 2 h and the solvent was evaporated. The solid product formed was collected, dried and finally crystallized from suitable solvent to afford 10a-i.
4-((Z)-2-Benzylidenehydrazinyl)-1-phenyl-3-((E)-styryl)-1H-pyrazolo[3,4-d]pyrimidine 10a:
Yellow crystals; m.p. 188˚C - 189˚C (acetonitrile); yield (87%); IR (KBr): υ = 3205 (NH) cm−1, 1H NMR (DMSO-d6): δ = 7.15 - 8.58 (m, 19H, Ar H), 12.04 (s, 1H, NH). MS: m/z (%) = 416 (M+, 14), 415 (42), 313 (43), 236 (41), 77 (100). Anal. for C26H20N6: Calcd. C, 74.98; H, 4.84; N, 20.18. found C, 74.37; H, 4.71; N, 19.73.
4-((Z)-2-(3-Methylbenzylidene)hydrazinyl)-1-phenyl-3-((E)-styryl)-1H-pyrazolo[3,4-d]pyrimidine 10b:
Yellow crystals; m.p. 192˚C - 194˚C (acetonitrile); yield (81%); IR (KBr): υ = 3352 (NH) cm−1, 1H NMR (DMSO-d6): δ = 2.36 (s, 3H, CH3), 7.24-8.52 (m, 18H, Ar H), 12.01 (s, 1H, NH); 13C NMR (DMSO-d6): δ = 20.83, 99.47, 119.11, 121.83, 125.20, 126.783, 128.18, 128.41, 128.76, 129.01, 130.57, 132.80, 136.52, 137.80, 138.19, 147.89, 153.98. MS: m/z (%) = 430 (M+, 79), 339 (52), 312 (100), 236 (79), 77 (80). Anal. for C27H22N6: Calcd. C, 75.33; H, 5.15; N, 19.52. found C, 75.06; H, 4.92; N, 19.36.
N,N-Dimethyl-4-((Z)-(2-(1-phenyl-3-((E)-styryl)-1H-pyrazolo[3,4-d]pyrimidin-4-yl)hydrazono)methyl)aniline 10c:
Yellow crystals; m.p. 242˚C - 244˚C (dimethylformamide); yield (77%); IR (KBr): υ = 3199 (NH) cm−1; 1H NMR (DMSO-d6): δ = 2.99 (s, 6H, N(CH3)2), 6.75-8.43 (m, 18H, Ar H), 11.87 (s, 1H, NH). MS: m/z (%) = 459 (M+, 34), 457 (62), 312 (100), 236 (65), 145 (60), 77 (91). Anal. for C28H25N7: Calcd. C, 73.18; H, 5.48; N, 21.34. found C, 72.55; H, 5.23; N, 20.84.
4-((Z)-(2-(1-Phenyl-3-((E)-styryl)-1H-pyrazolo[3,4-d]pyrimidin-4-yl)hydrazono)methyl)phenol 10d:
Yellow crystals; m.p. 270˚C - 272˚C (acetonitrile); yield (83%); IR (KBr): υ = 3324 (NH) cm−1; 1H NMR (DMSO-d6): δ = 6.99 - 8.68 (m, 18H, Ar H), 11.98 (s, 1H, NH), 13.02 (s, 1H, OH). MS: m/z (%) = 432 (M+, 82), 312 (100), 77 (93). Anal. for C26H20N6O: Calcd. C, 72.21; H, 4.66; N, 19.43. found C, 71.76; H, 4.51; N, 19.11.
4-((Z)-2-(4-Chlorobenzylidene)hydrazinyl)-1-phenyl-3-((E)-styryl)-1H-pyrazolo[3,4-d]pyrimidine 10e:
Yellow crystals; m.p. 230˚C - 232˚C (dimethylformamide); yield (84%); IR (KBr): υ = 3205 (NH) cm−1; 1H NMR (DMSO-d6): δ = 7.02 - 8.70 (m, 18H, Ar H), 12.11 (s, 1H, NH). MS: m/z (%) = 450 (M+, 100), 77 (72). Anal. for C26H19ClN6: Calcd. C, 69.25; H, 4.25; Cl, 7.86; N, 18.64. found C, 68.76; H, 4.03; Cl, 7.65; N, 18.23.
4-((Z)-2-(4-Fluorobenzylidene)hydrazinyl)-1-phenyl-3-((E)-styryl)-1H-pyrazolo[3,4-d]pyrimidine 10f:
Yellow crystals; m.p. 192˚C - 194˚C (acetonitrile); yield (87%); IR (KBr): υ = 3199 (NH) cm−1; 1H NMR (DMSO-d6): δ = 7.04 - 8.71 (m, 18H, Ar H), 12.13 (s, 1H, NH). MS: m/z (%) = 434 (M+, 87), 339 (32), 312 (100), 236 (79), 77 (56). Anal. for C26H19FN6: Calcd. C, 71.88; H, 4.41; F, 4.37; N, 19.34. found C, 71.51; H, 4.30; F, 4.29; N, 19.03.
1-Phenyl-3-((E)-styryl)-4-((Z)-2-(thiophen-2-ylmethylene)hydrazinyl)-1H-pyrazolo[3,4-d]pyrimidine 10g:
Yellow crystals; m.p. 186˚C - 188˚C (acetonitrile); yield (83%); IR (KBr): υ = 3337 (NH) cm−1; ¹H NMR (DMSO-d6): δ = 7.01 - 8.71 (m, 17H, Ar H), 12.01 (s, 1H, NH). MS: m/z (%) = 422 (M+, 100), 312 (88), 236 (61), 77 (42). Anal. for C24H18N6S: Calcd. C, 68.23; H, 4.29; N, 19.89; S, 7.59. found C, 67.81; H, 4.20; N, 19.64; S, 7.50.
1-Phenyl-4-((Z)-2-(pyridin-4-ylmethylene)hydrazinyl)-3-((E)-styryl)-1H-pyrazolo[3,4-d]pyrimidine 10h:
Yellow crystals; m.p. 284˚C - 286˚C (dimethylformamide); yield (84%); IR (KBr): υ = 3322 (NH) cm−1; 1H NMR (DMSO-d6): δ = 6.89 - 8.64 (m, 18H, Ar H), 11.97 (s, 1H, NH). MS: m/z (%) = 417 (M+, 54), 312 (71), 77 (100). Anal. for C25H19N7: Calcd. C, 71.93; H, 4.59; N, 23.49. found C, 71.58; H, 4.24; N, 23.11.
4-((Z)-2-(Benzo[d][1,3]dioxol-5-ylmethylene)hydrazinyl)-1-phenyl-3-((E)-styryl)-1H-pyrazolo[3,4-d]pyrimidine 10i:
Yellow crystals; m.p. 214˚C - 216˚C (acetonitrile); yield (82%); IR (KBr): υ = 3341 (NH) cm−1; 1H NMR (DMSO-d6): δ = 6.09 (s, 2H, CH2), 6.97 - 8.47 (m, 17H, Ar H), 12.01 (s, 1H, NH). MS: m/z (%) = 460 (M+, 54), 312 (100), 236 (63), 77 (92). Anal. for C27H20N6O2: Calcd. C, 70.42; H, 4.38; N, 18.25. found C, 70.01; H, 4.30; N, 18.11.
3.8. General Method for Preparation of 7H-Pyrazolo[4,3-e][1,2,4]Triazolo[4,3-c]Pyrimidine 11a-h
To a solution of an appropriate arylhydrazinyl compound 10a-h (5 mmol) in ethanol (20 mL), ferric chloride (4 mL, 2 M) was added, and the reaction mixture was stirred for 24 h. The solid that separated was collected, dried and finally crystallized from dimethylformamide to afford the corresponding 7H-pyrazolo[4,3-e][1,2,4]triazo- lo[4,3-c]pyrimidine 11a-h.
(E)-3,7-Diphenyl-9-styryl-7H-pyrazolo[4,3-e][1,2,4]triazolo[4,3-c]pyrimidine 11a:
White crystals; m.p. 276˚C - 278˚C; yield (87%); 1H NMR (DMSO-d6): δ = 7.37 - 8.81 (m, 17H, Ar H), 9.32 (s, 1H, NCH). MS: m/z (%) = 414 (M+, 100), 77 (80). Anal. for C26H18N6: Calcd. C, 75.35; H, 4.38; N, 20.28. found C, 75.03; H, 4.32; N, 20.01.
(E)-7-Phenyl-9-styryl-3-(m-tolyl)-7H-pyrazolo[4,3-e][1,2,4]triazolo[4,3-c]pyrimidine 11b:
White crystals; m.p. 182˚C - 184˚C; yield (86%); 1H NMR (DMSO-d6): δ = 2.23 (s, 3H, CH3), 7.31 - 8.47 (m, 16H, Ar H), 9.08 (s, 1H, NCH). MS: m/z (%) = 428 (M+, 100), 77 (26). Anal. for C27H20N6: Calcd. C, 75.68; H, 4.70; N, 19.61. found C, 75.22; H, 4.410; N, 19.15.
(E)-N,N-Dimethyl-4-(7-phenyl-9-styryl-7H-pyrazolo[4,3-e][1,2,4]triazolo[4,3-c]pyrimidin-3-yl)aniline 11c:
Yellow crystals; m.p. 222˚C - 224˚C; yield (85%); 1H NMR (DMSO-d6): δ = 3.02 (s, 6H, N(CH3)2), 6.89 - 8.41 (m, 16H, Ar H), 9.24 (s, 1H, NCH). MS: m/z (%) = 457 (M+, 47), 77 (100). Anal. for C28H23N7: Calcd. C, 73.50; H, 5.07; N, 21.43. found C, 73.21; H, 4.98; N, 21.24.
(E)-4-(7-Phenyl-9-styryl-7H-pyrazolo[4,3-e][1,2,4]triazolo[4,3-c]pyrimidin-3-yl)phenol 11d:
Pale green crystals; m.p. 284˚C - 286˚C; yield (88%); 1H NMR (DMSO-d6): δ = 6.93 - 8.45 (m, 16H, Ar H), 9.28 (s, 1H, NCH), 13.11 (s, 1H, OH). MS: m/z (%) = 430 (M+, 18), 77 (100). Anal. for C26H18N6O: Calcd. C, 72.55; H, 4.21; N, 19.52. found C, 72.31; H, 4.16; N, 19.39.
(E)-3-(4-Chlorophenyl)-7-phenyl-9-styryl-7H-pyrazolo[4,3-e][1,2,4]triazolo[4,3-c]pyrimidine 11e:
Yellow crystals; m.p. 250˚C - 252˚C; yield (80%); 1H NMR (DMSO-d6): δ = 7.33 - 8.12 (m, 16H, Ar H), 8.66 (s, 1H, NCH). MS: m/z (%) = 448 (M+, 100), 77 (48). Anal. for C26H17ClN6: Calcd. C, 69.56; H, 3.82; Cl, 7.90; N, 18.72. found C, 69.14; H, 3.75; Cl, 7.78; N, 18.59.
(E)-3-(4-Fluorophenyl)-7-phenyl-9-styryl-7H-pyrazolo[4,3-e][1,2,4]triazolo[4,3-c]pyrimidine 11f:
White crystals; m.p. 228˚C - 230˚C; yield (81%); 1H NMR (DMSO-d6): δ = 7.34 - 8.14 (m, 16H, Ar H), 8.81 (s, 1H, NCH). MS: m/z (%) = 432 (M+, 100), 77 (21). Anal. for C26H17FN6: Calcd. C, 72.21; H, 3.96; F, 4.39; N, 19.43. found C, 71.43; H, 3.90; F, 4.32; N, 19.31.
(E)-7-Phenyl-9-styryl-3-(thiophen-2-yl)-7H-pyrazolo[4,3-e][1,2,4]triazolo[4,3-c]pyrimidine 11 g:
Green crystals; m.p. 262˚C - 264˚C; yield (84%); 1H NMR (DMSO-d6): δ = 7.33 - 8.73 (m, 15H, Ar H), 9.48 (s, 1H, NCH); 13C NMR (DMSO-d6): δ = 98.57, 118.90, 122.25, 126.39, 126.81, 127.59, 128.51, 128.88, 129.24, 129.72, 135.62, 136.38, 137.73, 138.69, 142.28, 144.87, 145.56. MS: m/z (%) = 420 (M+, 100), 77 (18). Anal. for C24H16N6S: Calcd. C, 68.55; H, 3.84; N, 19.99; S, 7.63. found C, 68.01; H, 3.80; N, 19.51; S, 7.52.
(E)-7-Phenyl-3-(pyridin-4-yl)-9-styryl-7H-pyrazolo[4,3-e][1,2,4]triazolo[4,3-c]pyrimidine 11 h:
Orange crystals; m.p. 268˚C - 270˚C; yield (83%); ¹H NMR (DMSO-d6): δ = 6.91 - 8.68 (m, 16H, Ar H), 9.36 (s, 1H, NCH). MS: m/z (%) = 415 (M+, 100), 77 (41). Anal. for C25H17N7: Calcd. C, 72.28; H, 4.12; N, 23.60. found C, 71.74; H, 4.06; N, 23.31.
3.9. General Methods for Preparation of Pyrazolo[4,3-e][1,2,4]Triazolo[1,5-c]Pyrimidine Derivatives 13
Method A: To a solution of the appropriate 11a,e (1 mmol) in absolute ethanol (30 mL), sodium acetate (0.16 g, 2 mmol) was added and the mixture was refluxed for 8 h. The precipitated solid after cooling was filtered, washed with water, dried and finally crystallized from suitable solvent to give the respective products 13a,e.
Method B: To a solution of the compound 4 (1.64 g, 5 mmol) in pyridine (20 mL), the appropriate acid chloride 12a,e,j,k,l (5 mmol) was added. The reaction mixture was refluxed for 4 h, then cooled and poured over crushed ice containing hydrochloric acid (10%) with stirring. The solid product was filtered, washed with water, dried and finally crystallized from suitable solvent to afford 13a,e,j,k,l.
(E)-2,7-Diphenyl-9-styryl-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidine 13a:
Pale brown crystals; m.p. 210˚C - 212˚C (dimethylformamide); yield (80%); 1H NMR (DMSO-d6): δ = 6.66 - 8.75 (m, 17H, Ar H), 9.78 (s, 1H, NCH). MS: m/z (%) = 414 (M+, 100), 77 (28). Anal. for C26H18N6: Calcd C, 75.35; H, 4.38; N, 20.28. found C, 74.98; H, 4.18; N, 19.89.
(E)-2-(4-Chlorophenyl)-7-phenyl-9-styryl-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidine 13e:
Pale yellow crystals; m.p. 268˚C - 270˚C (dimethylformamide); yield (87%); 1H NMR (DMSO-d6): δ = 6.68 - 8.84 (m, 16H, Ar H), 9.81 (s, 1H, NCH). MS: m/z (%) = 448 (M+, 100), 447 (68), 77 (54). Anal. for C26H17ClN6: Calcd C, 69.56; H, 3.82; Cl, 7.90; N, 18.72. found C, 69.31; H, 3.66; Cl, 7.69; N, 18.40.
(E)-2-Methyl-7-phenyl-9-styryl-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidine 13j:
White crystals; m.p. 186˚C - 187˚C (acetic acid); yield (92%); 1H NMR (DMSO-d6): δ = 2.57 (s, 3H, CH3), 7.32 - 8.59 (m, 12H, Ar H), 9.50 (s, 1H, NCH); 13C NMR (DMSO-d6): δ = 14.28, 118.81, 122.05, 126.82, 127.37, 128.54, 128.88, 129.22, 135.60, 136.37, 137.87, 140.16, 144.17, 164.41. MS: m/z (%) = 352 (M+, 98), 351 (100), 77 (35). Anal. for C21H16N6: Calcd. C, 71.58; H, 4.58; N, 23.85. found C, 71.01; H, 4.33; N, 23.52.
(E)-2-(4-Bromophenyl)-7-phenyl-9-styryl-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidine 13k:
Pale yellow crystals; m.p. 290˚C - 292˚C (dimethylformamide); yield (85%); 1H NMR (DMSO-d6): δ = 6.71 - 8.80 (m, 16H, Ar H), 9.84 (s, 1H, NCH). MS: m/z (%) = 492 (M+, 100), 491 (75), 206 (30), 102 (29), 77 (81). Anal. for C26H17BrN6: Calcd C, 63.30; H, 3.47; Br, 16.20; N, 17.03. found C, 63.01; H, 3.39; Br, 16.03; N, 16.86.
(E)-2-(Furan-2-yl)-7-phenyl-9-styryl-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidine 13l:
Pale yellow crystals; m.p. 264˚C - 266˚C (dimethylformamide); yield (86%); ¹H NMR (DMSO-d6): δ = 6.75 - 8.77 (m, 15H, Ar H), 9.62 (s, 1H, NCH). MS: m/z (%) = 404 (M+, 100), 403 (65), 77 (53). Anal. for C24H16N6O: Calcd C, 71.28; H, 3.99; N, 20.78. found C, 70.79; H, 3.63; N, 20.47.
NOTES
*Corresponding author.