Hydrogen Engine and Numerical Temperature-Entropy Chart for Hydrogen/Air Cycle Analysis ()
1. Introduction
Hydrocarbon fuels have long been played a primary role in propulsion and power generation. Increase in stringent regulations on environment from exhaust emissions and future predictions for depletion of world petroleum reserves give strong impetus for research in the areas of alternative fuels and novel propulsion engines. Various alternative fuels such as LPG (Liquefied Petroleum Gas), Alcohols (Methanol and Ethanol), NG (Natural Gas), Biodiesel and Bio gasoline, and H2 (Hydrogen) have been given as substitutes for conventional hydrocarbon to reduce exhaust emissions [1] . Report [1] also highlighted and presented the novel propulsion engine at present time. Among these alternative fuels, hydrogen is considered as the near future, the long term renewable, sustainable, recyclable and non-polluting fuel [2] . Hydrogen-fueled internal combustion engine (ICE) is considered as the bridging technology from gasoline and diesel engines into the fuel cell engines. Significance is being attached to hydrogen in view to its CO2 free combustion, its properties and due to the fact that it can be produced from various feed stocks including renewable energy sources [3] . Though nitrogen oxides (NOx) emissions are formed upon hydrogen combustion, it can be minimized to low concentrations compared with hydrocarbon fuels [4] .
2. Combustion Properties of Hydrogen
The following are some important characteristics of hydrogen which affect the technical development of hydrogen internal combustion engines.
2.1. Flammability Range
Hydrogen has a wide flammability range (4.0% - 75% compared to 1.4% - 7.6% by volume in air for gasoline). This is of utmost importance for safe handling of hydrogen. However, this leads to the suitability for use in lean burn mixture engine. Lean mixture allows for better complete combustion and better thermal efficiency i.e. better economy. This in turn permits for lower for lower maximum combustion temperature that leads to lower nitrogen oxides (NOx) [3] [5] .
2.2. Quenching Distance
Hydrogen has a small quenching distance of 0.6 mm compared to 2.0 mm for gasoline. This is the distance from the cylinder wall where flame front extinguishes. This means it is more difficult to extinguish the hydrogen flame and implies more susceptibility of the engine to backfire since the hydrogen-air mixture flame more easily passes through valves than the gasoline-air mixture flame [3] [6] .
2.3. Flame Speed
Hydrogen burns with high flame front speed. This allows hydrogen engines to thermodynamically more closely approach the constant volume heat addition ideal cycle. This resemblance is adequate for stoichiometric mixture, when hydrogen engine runs lean to improve fuel economy and reduce nitrogen oxides, the flame speed slows down. However, it is still very much higher than the gasoline-air mixture flame speed [3] [6] . Flame speed and maximum combustion temperature are of prime concern for thermal efficiency and emissions.
2.4. Auto-Ignition Temperature
Self-ignition temperature of hydrogen is high as 585˚C. This makes it difficult to ignite the hydrogen-air mixture without an external ignition source. Table 1 shows this temperature compared to gasoline and diesel fuels. The table also summarizes the other important properties. The auto-ignition temperature is an important property in determining the maximum compression ratio (Higher Useful Compression Ratio) that an engine can operate at [7] .
2.5. Minimum Ignition Energy
The minimum ignition energy required to ignite the fuel-air mixture by an ignition source such as a spark plug for hydrogen is about an order of magnitude lower than gasoline-air mixture i.e. 0.02 mJ compared to 0.24 mJ for gasoline. The low ignition energy means that hot gases and hot spots on cylinder serve as sources of ignition creating premature ignition and flashback [3] [6] .
![]()
Table 1. Properties of hydrogen and conventional fuels.
2.6. Stoichiometric Air-Fuel Ratio and Mixture Energy Content
The stoichiometric combustion equation for hydrogen in air is:
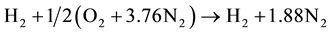 (1)
(1)
Stoichiometric air/fuel ratio is = 8/0.23 = 35 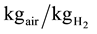
The excess air factor
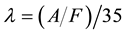 (2)
(2)
MOLS of air/MOL of hydrogen for complete combustion = 2.38.
This corresponds to stoichiometric volume percent of hydrogen in air-fuel mixture = 29.5.
For gasoline the volume percent of fuel in stoichiometric fuel-air mixture is only 1.76, with (A/F)st = 14.6.
Despite the lower calorific value of hydrogen is 120 mJ/kg compared to the gasoline as 44 mJ/kg, the energy content per m3 of gas fuel-air mixture under standard atmospheric conditions is lower [8] . Based on flammability range by volume in air, the flammability limits of hydrogen vary from equivalence ratio φ from 0.1 to 7.1. This enables the engine to run well into the lean mixture and prompts ignition and hence higher thermal efficiency [9] . Table 1 compares the properties of hydrogen and gasoline and diesel fuels.
2.7. Low Density
Without significance compression or conversion to liquid hydrogen, a very large volume may be necessary to store enough hydrogen to provide adequate drive range. It also implies the fuel-air mixture has low energy per unit mixture volume which reduces the engine output for carburetor or inlet port fuel induction. Thus, when hydrogen engine run lean issues of inadequate power arise [3] .
2.8. Configurations of Hydrogen Induction in ICE
In spark ignition engine, hydrogen can be used as fuel almost similar to SI gasoline engine. There are now many companies start marketing hydrogen ICE engine vehicles e.g. Ford and BMW [9] . The fuel induction modes are carburetion, inlet manifold and inlet port injection and direct cylinder injection [10] [11] . Hydrogen cannot be used as a sole fuel in CI engine due to the high self-ignition temperature of hydrogen. Hydrogen fueling of diesel engine must have spark plug in addition to hydrogen injector [12] .
3. Models for H2 Four Stroke Engine Cycle
The flame speed of hydrogen-air mixture is very high so that the ideal theoretical reference cycle would be the constant volume heat addition cycle. Also, to prevent excessive temperature inside the engine cylinder, the excess air factor λ must be higher than stoichiometric (preferable set to 1.5 - 2). The maximum end temperature Tgobtained upon combustion of H2-air mixture at constant volume, for fuel lower calorific value of 120 mJ/kg is:
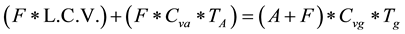 (3)
(3)
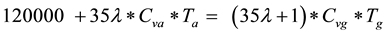 (4)
(4)
Taking Cva = 0.718 kJ/kg˚K, Cvg = 1.0 kJ/kg˚K is given in the following Table 2.
3.1. Ideal Gas Standard Cycle (Figure 1)
The working fluid in this cycle is assumed as air with constant specific heats with no fuel combustion but with equivalent amount of heat transferred through the cylinder wall. The specific heat ratio is taken as 1.4 and the compression ratio is 8. Assuming T1 = 400˚ K, P1 = 1 bar.
The indicated thermal efficiciencyand the indicated mean effective pressure are obtained from Equations (5) and (6) and results in Table 3.
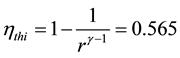 , (5)
, (5)
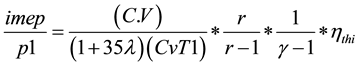 (6)
(6)
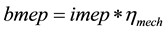 .
.
3.2. Generation of Temperature-Entropy-Energy Chart for H2
The equation for combustion of H2 with excess air factor λ can be written as:
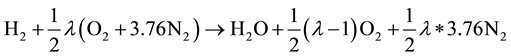 (7)
(7)
- Number of moles before combustion is given by:
![]()
Table 2. Maximum temperatures for different λ values.
![]()
Table 3. Variation of bmep & imep for different λ.
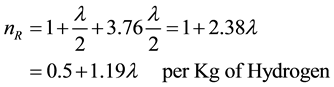 (8)
(8)
- Number of moles after combustion is given by:
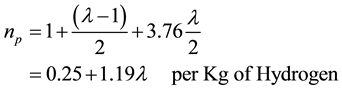 (9)
(9)
The calorific value is then L.C.V. = 120 MJ/kg hydrogen
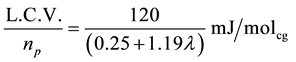
The following table shows (Table 4) ![]() for different values of (λ).
for different values of (λ).
The products of combustion are shown in Table 5:
The molar specific heat of each constitutes of the combustion gases as function of temperature (T) [8] . This is based on polynomial curve fit to thermodynamic data assuming that the unburned mixture is frozen in composition and the burned mixture is in equillibrium. This is based on the equilibrium NASA program [12] .
![]() (10)
(10)
Similar terms for![]() , and
, and ![]() as function of temperature.
as function of temperature.
As:![]() (11)
(11)
These specific heats of products of combustion are also function of (λ), the combustion gases molar specific heats at every temperature can be obtained in KJ/Kmolcg.˚K as:
![]() (12)
(12)
If the reference state for internal energy ![]() and enthalpy
and enthalpy ![]() is taken as
is taken as![]() ,
, ![]() at T0 = 0˚ K
at T0 = 0˚ K
The internal energy and enthalpy can be obtained at different temperatures and different excess air factor λ as:
![]() , (13)
, (13)
![]()
Table 4. (L.C.V. per molcg) for different λ values.
![]()
Table 5. Products of combustion of hydrogen.
The entropy at any given temperature and excess air factor λ also can be obtained if the reference state point is taken as:
![]() at P0 = 1 bar, T0 = 300˚ K and
at P0 = 1 bar, T0 = 300˚ K and![]() , so that,
, so that,
![]() (14)
(14)
![]() (15)
(15)
MATLAB is used in programmed mode to construct the temperature-entropy ![]() chart with constant pressure lines and constant specific volume lines, for different excess air factors λ, s. The temperature-internal energy lines
chart with constant pressure lines and constant specific volume lines, for different excess air factors λ, s. The temperature-internal energy lines ![]() and the temperature-enthalpy lines
and the temperature-enthalpy lines ![]() are also constructed on the chart for different excess air factors λ, s. All properties are given per mole combustion gases for reactants and products of combustion. The constructed temperature-entropy chart is shown in Figure 2 with some selected pressure and specific volumes lines and selected excess air factors.
are also constructed on the chart for different excess air factors λ, s. All properties are given per mole combustion gases for reactants and products of combustion. The constructed temperature-entropy chart is shown in Figure 2 with some selected pressure and specific volumes lines and selected excess air factors.
3.3. Fuel-Air Cycle Analysis for Hydrogen
In this analysis, the actual fuel-air working fluid is used and the combustion is assumed to occur instantaneous at TDC. The change of specific heat of the reactants and products of combustion is taken into the analysis. The change of the number of molecules and dissociation effects are taken into account. However, as the mixture excess air factor is 1.5 or more, the effects of dissociation are minimal. As a demonstration to the numerical procedure, the hydrogen SI four stroke engine cycles is applied to the generated ![]() chart. The engine is four stroke, water cooled engine having compression ratio r of 8 with hydrogen mixture excess air factor λ of 1.5. The in cylinder gases condition at start of compression stroke is taken as P1 = 1 bar and T1 = 400˚ K. The isentropic compression from the known state point (1) to state point (2) with the known specific volume
chart. The engine is four stroke, water cooled engine having compression ratio r of 8 with hydrogen mixture excess air factor λ of 1.5. The in cylinder gases condition at start of compression stroke is taken as P1 = 1 bar and T1 = 400˚ K. The isentropic compression from the known state point (1) to state point (2) with the known specific volume ![]() from the specified engine compression ratio, is iterated using Equation (15) for an excess air factor λ = ∞ and initial guess of T2. The temperature T2 is incremented until
from the specified engine compression ratio, is iterated using Equation (15) for an excess air factor λ = ∞ and initial guess of T2. The temperature T2 is incremented until![]() . At state (2) combustion carried out instantaneous and the working fluid changes into products of combustion at λ = 1.5. The lower calorific value is =
. At state (2) combustion carried out instantaneous and the working fluid changes into products of combustion at λ = 1.5. The lower calorific value is =
![]() . Assuming the cooling water percent loss as 34%, As distinct from gasoline or
. Assuming the cooling water percent loss as 34%, As distinct from gasoline or
diesel fuel/air cycles , all heat of combustion is added at constant volume due excessively high flame speed.
The internal energy of combustion gases ![]() is obtained from
is obtained from![]() . The maximum cycle temperature is determined from
. The maximum cycle temperature is determined from ![]() and the internal energy vector of combustion gases at different temperatures for λ = 1.5 generated from Equation (13). For the constant specific volume line and known temperature determine the specific entropy per mole combustion gases at state (3). Isentropic expansion from state (3) to state (4) with known specific volume
and the internal energy vector of combustion gases at different temperatures for λ = 1.5 generated from Equation (13). For the constant specific volume line and known temperature determine the specific entropy per mole combustion gases at state (3). Isentropic expansion from state (3) to state (4) with known specific volume ![]() is iterated using Equation (15) for excess air factor λ = 1.5 and an initial guess lower than T4 lower than T3. With the temperature T4 progressively decreased until
is iterated using Equation (15) for excess air factor λ = 1.5 and an initial guess lower than T4 lower than T3. With the temperature T4 progressively decreased until![]() . The properties of gases at state (4) is determined to obtain the exhaust gas loss, from which then, the indicated work done per cycle is determined. Engine sizing and performance parameters are predicted.
. The properties of gases at state (4) is determined to obtain the exhaust gas loss, from which then, the indicated work done per cycle is determined. Engine sizing and performance parameters are predicted.
The hydrogen/air cycle numerical representation is shown in the Figure 2 for engine compression ratio of 8 and λ = 1.5. The procedure is summerized as follows:
Point (1)
P1 =1bar, T1=400˚ K, ![]()
![]()
Point (2) Compression is isentroic from state (1) to (2) where ![]()
P2 = 13.75 bar, T2 = 767˚ K, Then, ![]()
T3 = 1883˚ K
![]()
Heat lost in exhaust = 35,238 ? 8572 = 26,666 kJ/molcg
IWD = 38,919 ? 26,666 = 12,253 kJ/molcg
The indicated thermal efficiency:
![]() ,
, ![]() , the indicated mean effective pressure
, the indicated mean effective pressure
![]() , brake mean effective pressure.
, brake mean effective pressure.
bmep = 421.1 * 0.87 = 366.33 kpa, where ηmechanical = 0.87
brake specific fuel consumption (bsfc) = ![]()
If the engine develops 50 kW @ N = 5000 rpm, L/D = 1 bmep =![]() , Vs = 3.27 liters with L = D =
, Vs = 3.27 liters with L = D =
10.1 cm. Pb/liters = 15.3 (which is low compared to modern gasoline engines).
The fuel/air cycle analysis of performance predictions compares very well with the actual engine cycles for petrol and diesel engine using hydrocarbon fuels [8] . The results obtained in the present work for hydrogen/air cycle analysis show a good estimate of engine sizing power developed and bsfc compared to that of actual engine. Also peak pressures and exhaust temperatures that affect engine structure and design can be closely predicted. The effect of many variables on hydrogen engine performance can also be understood.
For a certain engine configuration, CFD code Fluent which include the turbulence and detailed combustion processes that are modeled with sufficient generality to include delay period, chemical kinetics and flame propagation is then required. This simulation will give good understanding of in cylinder gas motion with detailed combustion process that are essential to improve performance and reduce emissions without sacrificing fuel economy and to study the effects of using different hydrogen fuel induction methods [13] .
4. Conclusion
Chemical and combustion properties of hydrogen are highlighted and proved to be the near future and the long term sustainable non-polluting fuel. Numerical procedure of the hydrogen/air engine cycle analysis with the constructed temperature-entropy chart for the reactants and products of combustion at different excess air factors, carried out for engine design and engine performance prediction. The hydrogen fueled SI engine performance prediction at excess air factor of 1.5 shows very low bsfc and relatively low volume specific power compared with conventional modern gasoline engine.
![]()
Figure 2. F/A cycle representation on temperature-entropy chart.
Nomenclature
![]() : Polynomial coefficients for i-gas constituents.
: Polynomial coefficients for i-gas constituents.
A: Fuel flow rate (kg/hr).
![]() ,
, ![]() ,
,![]() : Molar specific heat at constant pressure of i-gas constituents, combustion gases and air (kJ/mol.˚K).
: Molar specific heat at constant pressure of i-gas constituents, combustion gases and air (kJ/mol.˚K).
![]() ,
, ![]() ,
,![]() : Molar specific heat at constant pressure of i-gas constituents, combustion gases and air (kJ/mol・˚K).
: Molar specific heat at constant pressure of i-gas constituents, combustion gases and air (kJ/mol・˚K).
D: Cylinder bore (cm).
F: Fuel flow rate (kg/hr).
(A/F): Mass air-fuel ratio.
![]() : Molar specific enthalpy (kJ/mol・˚K).
: Molar specific enthalpy (kJ/mol・˚K).
L: Piston stroke (cm).
L.C.V.: Lower calorific value (kJ/kg).
N: Engine rotation speed (rpm).
P: Absolute pressure (bar, kPa).
![]() Universal gas constant (8.314 kJ/mol・˚K).
Universal gas constant (8.314 kJ/mol・˚K).
![]() Molar specific entropy (kJ/mol・˚K).
Molar specific entropy (kJ/mol・˚K).
T: Absolute temperature (˚K).
![]() : Molar specific internal energy (kJ/mol・˚K).
: Molar specific internal energy (kJ/mol・˚K).
![]() : Molar specific volume (m3/mol).
: Molar specific volume (m3/mol).
ɣ: Specific heat ratio.
λ: Excess air factor.