Biosorptive Removal of Zinc from Aqueous Solution by Algerian Calotropis procera Roots ()
1. Introduction
Global developments directed towards making human life increasing comfortable have greatly increased industrialization and urbanization. However, this trend has damaged the environment alarmingly, mainly due to the generation of a large amount of hazardous waste and the pollution of usable surface water. The major pollutants in wastewater are heavy metals such as lead, zinc, copper, cadmium, mercury, chromium and arsenic. These metals accumulate in living tissues/organs and can cause accumulative poisoning and serious health problems such as cancer and brain damage [1] .
There are numerous methods which are currently employed to remove these metals from aqueous environment. Some of these methods are chemical precipitation and sludge separation, chemical oxidation or reduction, ion exchange, reverse osmosis, membrane separation, electrochemical treatment and evaporation. Biosorption as a wastewater bioremediation process has been found to be an economically feasible alternative for metal removal. This method offers the advantages of low operating cost, minimizing secondary pollution and high efficiency in wastes [2] [3] . The use of nonliving biomass in biosorption is more practical and advantageous because living biomass cells often require the addition of fermentation media which increases the biological oxygen demand or chemical oxygen demand in the effluent. In addition, non-living biomass is not affected by the toxicity of the metal ions, and they can be subjected to different chemical and physical treatment techniques to enhance their performance.
The aim of the present work was to study the effect of some environmental parameters such as solution pH, initial Zn(II) concentration and contact time on the ability of Calotropis procera roots to biosorb Zn(II) ions from aqueous solutions.. Calotropis procera (Asclepiadaceae), commonly known as swallow wart, Sodom apple or milk weed, is a glabrous or hairy laticiferous shrub or small tree, found in tropical and subtropical Asia and Africa [4] . The leaves are widely used in Nigeria for coagulation of milk in preparing cheese [5] and the roots are traditionally used in India to treat diarrhea, cough, skin diseases, rheumatism, as an expectorant and emetic [6] [7] .
2. Materials and Methods
2.1. Adsorbent
The roots of Calotropis procera studied in this paper were taken from the local shrub roots in Oued Ksiksous in province Bechar (South West of Algeria). These roots were washed with deionized water to remove any dirt. They were dried in an oven at 60˚C for two days. The dried roots were ground and sieved through a sieve of 100 µm and then stored in a container. The sample of roots powder was characterized by using infrared (FT-IR) and scanning electron microscopic (SEM) techniques.
2.2. Reagents
All chemicals used were of analytical grade. Stock standard solution of Zn2+ has been prepared by dissolving the appropriate amount of ZnSO4 in deionized water. This stock solution was then diluted to specified concentrations. The pH of the system was adjusted using reagent grade NaOH and HCl respectively. All plastic sample bottles and glassware were cleaned, then rinsed with deionized water and dried at 60˚C in a temperature controlled oven.
2.3. Instrumentation
The pH of all solution was measured by a TitraLab Instrument TIM800 Model pH meter. The adsorption experiments have been studied by batch technique using a thermostated shaker bath GFL-1083 Model. An Eppendorf 5702 Model digital centrifuge was used to centrifuge the samples. Zn(II) concentrations of solutions before and after adsorption were measured by using flame atomic absorption spectrophometer (Varian, SpectrAA-100, AAS).
The Fourier transform infrared (FT-IR) absorption spectra was recorded on KBr pressed pellets of the powdered sample in the range 4000 - 400 cm−1, using a Perkine-Elmer FTIR 2000 spectrophotometer.
Nanomorphology was characterized by scanning electron microscopy (SEM) which was carried out using Hitachi S-4800 equipped with energy dispersive spectrometry for chemical analysis (EDS) and operating at 15 kV acceleration voltage.
2.4. Adsorption Procedure
Adsorption measurements were determined by batch experiments. The effect of contact time on the biosorption capacity of Calotropis procera roots was studied in the range 1 - 360 min at an initial concentration of 100 mg/L. Adsorption kinetics was studied using an initial concentration of 100 mg/L with the adsorbent dosage of 0.1 g/10mL at pH 6.5. Adsorption isotherms were studied at various initial concentrations of Zn(II) ion in the range of 10 - 120 mg/L and the experiments were conducted at different constant temperatures in the range of 25˚C - 60˚C. The amount of Zn(II) adsorbed per unit mass of Calotropis procera roots was calculated by using the mass balance equation given in Equation (1) [8] .
 (1)
(1)
where qe is the maximum adsorption capacity in mg/g, C0 is the initial concentration and Ce is the concentration at equilibrium of Zn(II) solution in mg/L, V is the volume of the Zn(II) solution in mL and m is the mass of CP roots in grams.
The sorption capacity at time t, qt (mg/g) was obtained as Equation (2) [9] :
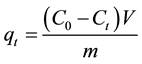 (2)
(2)
where C0 and Ct (mg/L) are the liquid phase concentrations of Zn(II) at initial and a given time t, V is the solution volume and m the mass of CP roots (g).
3. Results and Discussion
3.1. Characterisation of Adsorbent
Observation under a scanning electron microscope (SEM) in Figure 1 shows that grains of CP roots have amorphous structures; they are agglomerated in balls of different sizes, which can have cavities inside.
The FTIR Spectra of Calotropis procera roots, in the range of 400 - 4000 cm−1 was taken to confirm the presence of functional groups that might be responsible for the biosorption process. Peaks appearing in the spectrum (Figure 2) were assigned to various groups and bond in accordance with their respective wavenumber as reported in former literature. The region between 3100 cm−1 and 3600 cm−1 showed a broad and strong band stretch, indicative of the presence of −NH2 groups and free or hydrogen bonded O−H groups [10] [11] . The light stretch at 2928 cm−1 showed the asymmetric C−H vibration [12] . The peak at 1644 cm−1 was of COO−, C=O and C−N peptidic bond of proteins [12] . The peak at 1412 cm−1 was due to the symmetric bending of CH3 [13] . Band at 1236 cm−1 was assigned to C−O vibration.The bands at 1080 cm−1 might be phosphonate (P−OH stretching) [10] [14] . The bands at 770 cm−1 could be assigned to ester vibrations [15] . It was noted that the IR spectrum of Calotropis procera roots supported the presence of O−H, COOH, C=O, C−N, C−H, −NH2, C−O and POH as functional groups. The diversity of functional groups indicated the complex nature of the biomass examined. This was similar to the earlier reports on functional groups of biomass [10] [14] .
![]()
![]()
Figure 1. SEM of Calotropis procera roots.
![]()
Figure 2. Infrared (IR) spectra of Calotropis procera roots.
The displacements of the corresponding bands −OH, −NH and carboxyl groups indicate the involvement of these groups in the biosorption of heavy metals by the roots of Calotropis procera.
3.2. Effect of Initial Zn(II) Concentration on Metal Biosorption
The metal distribution between the biomass and the aqueous solution at equilibrium is of importance in determining the maximum biosorption capacity of the biomass for Zn(II) ions. The effect of initial metal concentration on the biosorption capacity was investigated at pH 6.5. In Figure 3, biosorption of Zn(II) increased much quickly with increasing initial metal concentration from 10 to 500 mg/l. A higher initial concentration provides an important driving force to overcome most partly of mass transfer resistance between the metal solution and Calotropis procera cell wall, and therefore the the biosorption capacity increases. In addition, the number of collisions between metal ions and the biosorbent increases with increasing initial metal concentration so the biosorption process is enhanced [16] . Biosorption rate was decreased with increasing initial concentration from 200 to 500 mg/l and this can be explained by the saturation of the biosorption sites on the biomass surface.
3.3. Effect of pH on Metal Biosorption
pH is an important parameter influencing heavy metal adsorption from aqueous solutions. It affects both the surface charge of adsorbent and the degree of ionization of the heavy metal in solution. The pH range of 1.5 - 6.5 was chosen, as the precipitation of Zn(II) is found to occur at pH ≥ 7 [17] . Variation of adsorption capacity of biomass for Zn(II) ions with pH is shown in Figure 4. The removal of metal ions from solution by adsorption is highly dependent on the pH of the solution. The biosorption of Zn(II) ions increases steadily with increase in intial pH and the maximum equilibrium biosorption capacity of 4.8 mg/g is observed at pH 6.5 (natural pH of suspension).
3.4. Effects of Interaction Time and Kinetics of Adsorption
The biosorption of Zn(II) on CP roots as a function of contact time at pH 6.5 is shown in Figure 5. The results indicated that the Zn(II) interacted with the biomass rapidly within the first 15 min. Afterwards, the interactions slowed down and reached equilibrium in 40 min.
Attainment of equilibrium is influenced by several factors including the nature of the adsorbent and the adsorbate, and the interactions between them. The kinetics of the interactions is thus likely to be dependent on different rate processes as the interaction time increases [18] .
![]()
Figure 3. Effect of initial concentration of Zn(II) on biosorption capacity of Calotropis procera roots.
![]()
Figure 4. Effect of pH on biosorption of Zn(II) by Calotropis procera roots.
On the basis of this result, it can be observed that CP roots can be used to remove this metal ion.
Many models were used to describe the adsorption processes. The most appreciated was Pseudo second-order (Equation (3)) [19] :
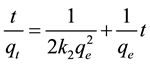 (3)
(3)
where qt (mg/g) is the amount of Zn(II) adsorbed at time t (min), qe (mg/g) is the equilibrium adsorption capacity and K2 (g/(mg・min)) is the pseudo-second-order rate constant of adsorption. The values of K2 and qe calculated from the intercept and slope of equation are 0.28 g/(mg・min) and 4.80 mg/g, respectively.
Figure 6 shows a linear plot with very high value of the correlation coefficient (R2 = 1), in addition to the good agreement between experimental and calculated values of qe. Therefore, the adsorption of Zn(II) onto CP roots is greatly represented by the pseudo-second-order kinetics. In many cases, the second-order equation correlates well to the adsorption studies [20] . The applicability of second-order to the adsorption data indicates that the concentration of both CP roots and Zn(II) ions are involved in the rate determining step. Similar trends were shown for the adsorption of Zn(II) onto kaolin [8] and onto chitosan derivatives [21] .
3.5. Construction of Isotherms and Model Fitting
Several adsorption isotherm models have been employed to interpret the adsorption behaviors of heavy metals on solid adsorbents. In this study, the data collected have been fitted to the Langmuir isotherm [22] and the
![]()
Figure 5. Effect of contact time on biosorption of Zn(II) by CP roots.
![]()
Figure 6. Lagergren pseudo second-order plots for Zn(II) biosorbed on CP roots.
Freundlich isotherm [23] , as described in Equations (4) and (5), respectively.
Langmuir equation
 (4)
(4)
Freundlich equation
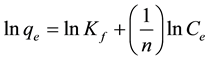 (5)
(5)
In these equations, Ce is the concentration of Zn(II) in solution (mg/L) at equilibrium, qe is the amount of the adsorbed metal ions (mg/g) at the solid/liquid interface, qmax is the monolayer capacity of the adsorbent (mg/g), KL is the Langmuir adsorption constant (L/mg), Kf and 1/n are empirical parameters, Kf is the adsorption constant related to the bonding energy and 1/n is associated to the surface heterogeneity.
The sorption isotherms of metal ions on CP roots were fitted by two models, as shown in Figure 7 and Figure 8. The parameters predicted by the two different models are summarized in Table 1. In general, parameters were fit using the linear adjustment and the correlation coefficient was fit better using the Langmuir model. The high value of R2 as 0.991 indicated minimal deviation from the fitted equation showing that the adsorption data would follow Langmuir equation. Also, the data in Table 1 indicated that the maximum adsorption capacity of CP roots for Zn(II) was calculated as 9.69 mg/g. It can be mentioned that the surface of CP roots is homoge- neous and the adsorption of Zn(II) formed a monolayer on its outer surface [24] . Agouborde et al. [25] also, found the adsorption of some heavy metals such as Zn(II) onto sawdust and Brine sediments followed Langmuir model and formed monolayer with monolayer capacity (qm) of 2.58 mg/g and 4.85 mg/g respectively. Pehlivan et al. [26] found qm = 0.176 mg/g with sugar beat pulp and qm = 11.11 mg/g with fly ash. Other authors have reported a monolayer capacity of 1.66 mg/g for the adsorption of Zn(II) by Low rank Turkish coal [27] and 8.64 mg/g by Granite [28] . It can be seen that CP roots are an effective adsorbent for Zn(II), when compared with some other adsorbents.
4. Conclusion
Biosorption technology, utilizing natural materials for effectively removing metals from aqueous media, offers an efficient alternative compared to traditional chemical and physical treatments. The goal of this work was to explore the potential use of Calotropis procera roots as a low-cost sorbent for removing Zn(II) ions from
![]()
Figure 7. Langmuir isotherm plot for biosorption of Zn(II) on CP roots.
![]()
Figure 8. Freundlich isotherm plot for biosorption of Zn(II) on CP roots.
![]()
Table 1. Langmuir and Freundlich parameters for biosorption of zinc on CP roots.
aqueous solutions by batch design. The adsorption was found to be drastically depending on initial metal ion concentration, contact time and pH solution. The results gained from this study were well described by Langmuir model with monolayer capacity qm = 9.69 mg/g. Calotropis procera roots had a high adsorption capacity when compared with some other adsorbents reported in literature. This adsorption can be a good choice for removal of not only Zn(II) ions but also other heavy metal ions from waste water stream.
NOTES
*Corresponding author.