1. Introduction
Lead is one of the heavy metals that are often found in industrial wastewater and its discharge into the environment poses serious threat due to its toxicity to aquatic and terrestrial lives [1] [2] . It is a group IV element on the periodic table which is remarkably highly resistant to corrosion in most acid and naturally occur as element buried in the earth crust in insoluble and biologically inoffensive forms [3] . Enhanced industrialization such as manufacturing of storage batteries, television tube, printing, paints, pigments, photographic materials, gasoline additives, matches and explosives brought about lead bearing wastewater [4] - [7] .
Exposure to Lead is widely recognized as a major risk factor for several human diseases once it goes beyond the World Health Organisation (WHO) maximum permissible limit (3 - 10 mg・L−1) in drinking water [8] - [12] . It forms complexes with oxo-groups in enzymes to affect virtually all steps in the process of haemoglobin synthesis and porphyrin metabolism [13] . Other problems associated with toxic levels of lead exposure are encephalopathy, seizures and mental retardation, anemia and nephropathy [14] [15] . Hence, lead must be removed as much as possible from industrial effluents to prevent environmental hazard from its discharge.
Adsorption technology has been widely preferred to other traditional methods such as coagulation, flocculation, filtration, ozonation or sedimentation in the removal of pollutants from wastewater [16] - [18] . In this technology, activated carbons are commonly used adsorbent due to their high adsorption capacity, a result of their high surface area and surface reactivity but their regeneration is, however, difficult and expensive [19] [20] .
Quest for effective and economical technologies have brought about biosorption. It is based on metal binding capacities of various biological materials mainly composed of cellulose, hemicelluloses and lignin that make them effective adsorbents for a wide range of pollutants due to the presence of functional groups such as hydroxyl, carboxyl, methoxyl and phenols [21] -[25] . Recent studies have shown that heavy metals can be removed using plant materials such as empty palm oil fruit bunch [26] , sour soup seeds [27] , modified cassava fibre [28] , coconut shell [29] , duck weed [30] , sago waste [31] , African spinach stalk [32] , palm fruit fibre [33] , hop [34] , orange peels [35] - [37] and spent tea leaves [18] [38] .
Banana Stalks (BS) are generated in large amount as waste product after the consumption of banana and these constitute environmental menace due to the fact that they are left to decompose being of non-economic importance [39] . However, BS is a rich lignocellulosic agricultural waste whose previous application has been limited to the production of activated carbon for malachite green dye removal [40] and not yet applied to Pb(II) removal [41] - [48] . The present study investigated the adsorptive capacity of BS as a low-cost adsorbent for the biosorption of Lead(II) from aqueous solution. The effects of physical parameters such as initial concentration, pH, and temperature and biosorbent dosage on the biosorption process have been investigated. In addition, the biosorption equilibrium isotherms, kinetics and thermodynamic parameters have been determined.
2. Materials and Method
2.1. Preparation and Characterization of Adsorbent
Banana Stalks were first sundried, cut into small pieces and washed with distilled water to remove dirt particles. BS pieces were then dried in the oven at 100˚C for 24 hours to constant weight before being grounded and screened to 300 - 425 mm mesh particle size. It was then stored as Raw Banana Stalk (RBS). RBS of 200 g was soaked with 1 M H3PO4 in ratio 1:1, kept in the oven for 24 hours at 80˚C for activation and stored as Acid Activated Banana Stalk (AABS). Similarly, Base Activated Banana Stalk (BABS) was prepared by soaking another 200 g of the RBS in 0.1 M KOH for 24 hours at 80˚C in an oven and stored as BABS. The Proximate analysis of the biosorbents was carried out to know the percentage compositions of its constituents. Fourier Transform Infrared (FTIR) spectroscopic analysis was performed on RBS, AABS and BABS using FTIR spectroscope (FTIR-2000, Perkin-Elmer). The spectra were measured from 4000 to 400 cm−1.
2.2. Preparation of Simulated Wastewater
Simulated wastewater samples containing Pb(II) was prepared from Pb(NO3)2 of 1000 mg・L−1 stock solutions. Reagents used were of analytical grade and deionized water was used in solution preparation. Other concentrations (20 - 100 mg/L) were obtained from this stock solution by serial dilution. Fresh dilutions were used for each experiment. The concentration of Pb(II) in simulated wastewater was analysed by Atomic Absorption Spectrophotometer (model PyeUnicam SP-9 Cambridge, UK).
2.3. Batch Biosorption Equilibrium Studies
Batch equilibrium tests were carried out on the adsorption of Pb(II) on RBS. The effect of initial metal ion concentration, contact time, temperature, solution pH and adsorbent dose were investigated. Sample solutions were withdrawn at time interval and equilibrium to determine residual concentrations. Solutions were filtered prior to analysis in order to minimise the interference of the RBS. For equilibrium studies, the experiment was carried out for 360 minutes to ensure that equilibrium was reached. The linear Beer-Lambert relationship between absorbance and concentration with the calibration curve was established by plotting the graph of absorbance versus concentration of the lead solution. The concentration of lead solution before and after adsorption was determined using an Atomic Absorption Spectrophotometer (model PyeUnicam SP-9 Cambridge, UK). This experiment was done in turns for AABS and BABS.
The adsorbed phase concentration ( , in mg/g) at time (t) was calculated using Equation (1)
, in mg/g) at time (t) was calculated using Equation (1)
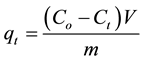 (1)
(1)
where,  and
and 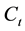 are the initial and the final lead concentration (mg/L), respectively;
are the initial and the final lead concentration (mg/L), respectively;  is the water sample volume (L); and m is the mass of adsorbent used (g).
is the water sample volume (L); and m is the mass of adsorbent used (g).
The biosorption at equilibrium, 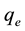 (mg/g), was calculated according to Equation (2)
(mg/g), was calculated according to Equation (2)
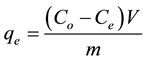 (2)
(2)
where,  and
and 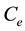 are the initial and the final (equilibrium) lead concentration (mg/L) respectively;
are the initial and the final (equilibrium) lead concentration (mg/L) respectively; 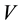 is the water sample volume (L); and m is the mass of adsorbent used (g). The percentage of lead ion removal was calculated using Equation (3):
is the water sample volume (L); and m is the mass of adsorbent used (g). The percentage of lead ion removal was calculated using Equation (3):
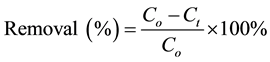 (3)
(3)
2.4. Effects of Adsorption Conditions
RBS of 1 g was measured into a 125 ml Erlenmeyer flask with 100 ml of 100 mg/l of Pb(II) solution with pH of 7 monitored using a pH meter (model CD70 WPA) and controlled with 0.1 M NaOH and 0.1 M HCl. The flask was agitated at 30˚C at a constant 125 rpm using the Thermostatic water bath shaker, the sorption experiment carried out for sufficient time of 360 minutes. The supernatant was filtered using Watman Filter Paper and Lead(II) concentration was analysed using Atomic Absorption Spectrophotometer (AAS). The effect of contact time (30, 60, 90, 120, 150, 180, 210, 240, 300, 360 minutes), biosorbent dose (0.2, 0.4, 0.6, 0.8, 1.0, 1.5 and 2.0 g), initial metal ion concentration (20, 40, 60, 80, 100 and 120 mg/L), pH (4, 6 and 8) and temperature (30, 40 and 50˚C) were evaluated during the study. During the experiment, the particular parameter considered was varied while the other four were kept constant variously for RBS, BABS and AABS biosorbents.
3. Results and Discussion
3.1. Characterisation of Biosorbent
The result of proximate analysis carried out on the biosorbent samples are as presented in Table 1. It revealed that the AABS, BABS and RBS are rich in crude fibre of 67.69%, 62.07% and 58.47%, respectively. This rich crude fibre comprise of lignin, cellulose and hemicellulose content. Lignin is a compound rich with functional groups, such as carbonyl, ether and hydroxyl [21] [41] and has been used for metals adsorption by some researchers [40] [49] . The implication of this is that the presence of the active site presupposes that banana stalks are good biosorbents. The FTIR spectra characteristics for the three biosorbents are as shown on Table 2 while main spectra are on Figure 1. The analysis revealed that the biosorbents are rich in hydroxyl, carboxyl and the phenolic groups which indicates that RBS, AABS and BABS are viable for the adsorption of Pb(II) onto the biosorbents.
3.2. Effect of Initial Concentration and Contact Time
The rate of biosorption is a function of the initial concentration and contact time of the sorbate and these makes them important factors for effective biosorption. Figure 2 shows the effect of contact time on Pb(II) adsorption onto RBS, BABS and AABS. It was found that the adsorption of Pb(II) by the three banana stalks increased with contact time until equilibrium was reached at around 180 minutes. The biosorption process was rapid initially
![]()
Table 1. Proximate analysis of banana stalks.
![]()
Table 2. FTIR spectra characteristics for RBS, BABS and AABS.
![]() (a)
(a)![]() (b)
(b)![]() (c)
(c)
Figure 1. FTIR Spectroscopy for the biosorbents, (a) RBS; (b) BABS; (c) AABS.
![]()
Figure 2. Effect of contact time on the percentage removal of Lead(II).
due to increased availability of active binding site on the biosorbent surface and this is controlled by diffusion process from the bulk to the surface. However, during the last stages, biosorption is likely to be attachment- controlled process due to reduction in the available active sites. AABS has 96.00% removal at equilibrium, followed by BABS (66.90%) while RBS had the least percentage removal and adsorptive capacity even beyond 180 minutes of 63.9% and 6.39 mg・g−1, respectively. Figure 3 reveals that, at lower concentrations of 20 - 40 mg・L−1, biosorption was completed at about 60 minutes while at higher concentration, the biosorption process became constant at 180 mins as observed in Figures 3(a)-3(c), respectively. At lower concentrations, all metal ions present in the solution interacted with binding sites and then facilitated about 70.1% on RBS (Figure 3(a)) 74.6% on BABS (Figure 3(b)) with 93% Pb(II) removal on AABS (Figure 3(c)). At higher concentrations between 80 - 120 mg・L−1, more Pb(II) is left unabsorbed in the solution due to the saturation of binding sites. This may be due to the increase in the number of ions competing for available binding sites on the three Banana Stalks considered in this study. This result is similar to other researcher findings [18] [50] .
3.3. Effect of Adsorbent Dose
Varying adsorbent dosage of 0.2 to 2.0 g were used to test for the effect of adsorbent dose on the biosorption process keeping the initial lead concentration at 100 mg・L−1, while all other parameters were kept constant. It was observed as shown on Figure 4 that, when the adsorbent dose was increased from 0.2 g to 2.0 g, the percentage adsorption generally increased from 30.2% to 97.3%, but the amount adsorbed per unit mass of adsorbent decreased considerably. The increase in the adsorption percentage or decrease in unit adsorption with increased dose of the biosorbent is due to the increase in active sites on the adsorbent and thus making easier penetration of the metal ions to the adsorption sites which is in agreement with previous researcher [33] . When the adsorbent added is beyond 0.6 g, the decrease in lead ion adsorption is not very prominent which is perhaps due to the formation of adsorbent agglomerates reducing available surface area and blocking some of the adsorption sites. Maximum adsorption capacity of lead ions was observed with adsorbent dose of 0.6 g with adsorption capacity of 15.8 mg・g−1 and thereafter, a slow increase in the percentage removal was seen reaching a constant value with respect to adsorbent dosage.
3.4. Effect of pH
Surface charges on the adsorbent and degree of ionization of the sorbate are affected by the pH of the solution and this makes pH and important factor in adsorptive studies. Figure 5 reveals that, the adsorptive capacity was best at pH 8 with 89% removal of the lead ion. This is because biosorption is low at strong acidic medium (pH 2 - 4) because the surface charge on the biosorbents is positive, thus, Pb(II) is not favourable attached because of electrostatic repulsion between the RBS, BABS and AABS surfaces and the positively charged Pb(II). At higher pH, uptake increased because more metal binding sites could be exposed and carried negative charges, with
![]() (a)
(a)![]() (b)
(b)![]() (c)
(c)
Figure 3. Effect of different initial lead concentration on the percentage removal of Pb(II) on Raw banana stalk biosorbent, (a) RBS; (b) BABS; (c) AABS.
![]()
Figure 4. Effect of adsorbent dose on the percentage removal of Pb(II) removal using different biosorbents.
subsequent attraction of the positively charged metal ions with the biosorbent surface and this agreed with Bello et al. [40] on the adsorptive features of banana stalk-based activated carbon for malachite green dye removal. Experiments were carried out with the pH values of up to 8 due to the fact that metal precipitation appeared at higher pH values and interfered with the accumulation or biosorbent deterioration which was in agreement with the result obtained by Puranik and Paknikar [51] .
3.5. Effect of Temperature
The percentage Pb(II) removal decreased with increased temperature for AABS as shown on Figure 6 while BABS and RBS followed a reverse trend. AABS highest percentage removal was at 30˚C (82.4%) and declined with increasing temperature. The reverse in the trend of result obtained for AABS compared with the other two may be due to the fact that at increased temperature, AABS surface for adsorption has acquire the maximum energy for adsorption while RBS and BABS had not. A similar result, in which adsorptive capacity is inversely proportional to temperature was obtained by Paresh et al. [52] using a biosorbent. Lower temperature of 30˚C also favoured the Biosorption of Lead(II) and Nickel(II) using Pigeon peas hulls waste by Ramana et al. [53] .
3.6. Adsorption Kinetics for Biosorption
3.6.1. The Pseudo-First-Order Kinetics Model
The pseudo-first-order kinetics equation is expressed as Equation (4) according to Lagergren [54]
![]() (4)
(4)
where ![]() and
and ![]() are the adsorption capacities at equilibrium (mg/g) and time
are the adsorption capacities at equilibrium (mg/g) and time![]() , respectively.
, respectively. ![]() is the rate constant for Pseudo-first-order adsorption (min−1). Plots of
is the rate constant for Pseudo-first-order adsorption (min−1). Plots of ![]() against
against ![]() at various temperatures resulted in linear graphs with negative slopes
at various temperatures resulted in linear graphs with negative slopes ![]() and
and ![]() values were calculated as intercepts. All the values as well as their correlation coefficient
values were calculated as intercepts. All the values as well as their correlation coefficient ![]() are as shown on Table 3 for biosorbents. Though values of
are as shown on Table 3 for biosorbents. Though values of ![]() were high but high SSE and the various disparity between the values of
were high but high SSE and the various disparity between the values of ![]() and
and ![]() is an indication that the biosorption of Pb(II) unto AABS, BABS and RBS do not follow the pseudo-first-order kinetics.
is an indication that the biosorption of Pb(II) unto AABS, BABS and RBS do not follow the pseudo-first-order kinetics.
3.6.2. The Pseudo-Second-Order Kinetics Model
The pseudo-second-order kinetics equation is expressed as Equation (5) according to Ho and Mckay [55]
![]()
Figure 5. Effect of pH on the percentage removal of Lead(II) using biosorbents.
![]()
Figure 6. Effect of temperature on the percentage removal of Pb(II) using different banana stalk biosorbents.
![]()
Table 3. Pseudo-first-order and pseudo-second-order kinetic parameters and correlation coefficients obtained for the biosorption of Pb(II).
![]() (5)
(5)
where ![]() is the rate constant of pseudo-second-order adsorption (g・mg−1・min−1). For the boundary conditions
is the rate constant of pseudo-second-order adsorption (g・mg−1・min−1). For the boundary conditions ![]() to
to ![]() and
and ![]() the integrated form of the equation becomes
the integrated form of the equation becomes
![]() (6)
(6)
Rearranging this equation, we have a linear form:
![]() (7)
(7)
A plot of ![]() versus
versus ![]() was made and
was made and ![]() and
and ![]() value was estimated from the slopes and intercepts of the plot. The Sum of Error Square (SSE%) was also used to evaluate the difference between the
value was estimated from the slopes and intercepts of the plot. The Sum of Error Square (SSE%) was also used to evaluate the difference between the ![]() and
and![]() , giving as
, giving as
![]() (8)
(8)
where, ![]() is the number of experiments.
is the number of experiments.
The ![]() values were as high as 0.99 and the sum of square error was lower when compared to that of pseudo-first-order as there was good agreement between the
values were as high as 0.99 and the sum of square error was lower when compared to that of pseudo-first-order as there was good agreement between the ![]() and
and ![]() of the pseudo-second-order kinetics as shown on Table 3. Therefore the biosorption of Pb(II) onto AABS,BABS and RBS follows a pseudo-second- order kinetics.
of the pseudo-second-order kinetics as shown on Table 3. Therefore the biosorption of Pb(II) onto AABS,BABS and RBS follows a pseudo-second- order kinetics.
3.7. Adsorption Isotherms AABS
3.7.1. Langmuir Isotherm Model
Langmuir isotherm is based on the monolayer sorption of Pb(II) on the surface of carbon sites and is represented linearly by the following equation [56] :
![]() (10)
(10)
where ![]() (mg/L) and
(mg/L) and ![]() (mg/g) are the concentration of Pb(II) solution (mg/L) and the amount of adsorbed lead at equilibrium, respectively,
(mg/g) are the concentration of Pb(II) solution (mg/L) and the amount of adsorbed lead at equilibrium, respectively, ![]() or
or ![]() and
and ![]() are Langmuir constants related to sorption capacity (mg/g) and the energy of sorption (L/mg) respectively.
are Langmuir constants related to sorption capacity (mg/g) and the energy of sorption (L/mg) respectively.
The essential characteristics of the Langmuir isotherm can be expressed in terms of a dimensionless equilibrium parameter ![]() which confirms the favourability of the adsorption process. Given as,
which confirms the favourability of the adsorption process. Given as,
![]() (11)
(11)
where ![]() is the Langmuir constant and
is the Langmuir constant and ![]() is the highest Pb(II) concentration (mg/L).
is the highest Pb(II) concentration (mg/L).
The value of ![]() indicates the type of the isotherm to be either unfavourable
indicates the type of the isotherm to be either unfavourable![]() , linear
, linear![]() , favourable
, favourable ![]() or irreversible
or irreversible![]() . The values of
. The values of ![]() reported in Table 4 obtained at various temperatures were <1, indicating that the adsorption of Pb(II) onto AABS is favourable. The value of
reported in Table 4 obtained at various temperatures were <1, indicating that the adsorption of Pb(II) onto AABS is favourable. The value of ![]() obtained was compared with those of other adsorbents used for Pb(II) removal from aqueous solution and the present study gave the best as shown on Table 5.
obtained was compared with those of other adsorbents used for Pb(II) removal from aqueous solution and the present study gave the best as shown on Table 5.
3.7.2. Freundlich Isotherm Model
Freundlich isotherm describes the heterogeneous surface energies by multilayer sorption and is expressed by the following equation [57] :
![]() (12)
(12)
![]()
Table 4. Comparison of the Isotherms for the adsorption of lead onto AABS at different temperatures.
![]()
Table 5. Comparison of adsorption capacities of various adsorbents for Pb(II).
Linearised as,
![]() (13)
(13)
where ![]() and
and ![]() are the Freundlich constants incorporating the factors affecting the adsorption capacity and the degree of non-linearity between the solute concentration in the solution and the amount adsorbed at equilibrium respectively. Plots of log
are the Freundlich constants incorporating the factors affecting the adsorption capacity and the degree of non-linearity between the solute concentration in the solution and the amount adsorbed at equilibrium respectively. Plots of log ![]() versus log
versus log ![]() gave linear graphs with high to
gave linear graphs with high to![]() . Comparing the
. Comparing the ![]() values of the two isotherms (Table 4), the adsorption fits the Langmuir model better. Value of
values of the two isotherms (Table 4), the adsorption fits the Langmuir model better. Value of ![]() indicates that the adsorption is favourable.
indicates that the adsorption is favourable.
3.7.3. Temkin Isotherm Model
Temkin isotherm based on the ions sorption heat, which is due to the sorbate and adsorbent interactions, is given by Temkin and Pyzhev [58]
![]() (14)
(14)
where:
![]() (15)
(15)
where ![]() is the concentration of sorbate at equilibrium (mg・L−1),
is the concentration of sorbate at equilibrium (mg・L−1), ![]() is the quantity of sorbate bioadsorbed at equilibrium (mg・g−1),
is the quantity of sorbate bioadsorbed at equilibrium (mg・g−1), ![]() is the equilibrium binding constant (L/mol) corresponding to the maximum binding energy and constant
is the equilibrium binding constant (L/mol) corresponding to the maximum binding energy and constant ![]() is related to the heat of sorption.
is related to the heat of sorption. ![]() is the temperature
is the temperature![]() , and
, and ![]() is the ideal gas constant (8.314 × 10−3 kJ・mol−1・K−1). The values of
is the ideal gas constant (8.314 × 10−3 kJ・mol−1・K−1). The values of![]() ,
, ![]() and
and ![]() are given in Table 4, the lower value of
are given in Table 4, the lower value of ![]() being 1.32 kJ・mol−1 (<8 kJ・mol−1) indicates that the interaction between lead ions and AABS can be expressed as a physisorption process.
being 1.32 kJ・mol−1 (<8 kJ・mol−1) indicates that the interaction between lead ions and AABS can be expressed as a physisorption process.
3.7.4. Dubinin-Radushkevich Isotherm (D-R) Model
The Dubinin-Radushkevich (D-R) model was used to estimate the characteristic porosity of the AABS and the apparent energy of adsorption. The non-linear form of the D-R isotherm equation is given in Equation (16) according to Dubinin-Rasdushkevich [59] :
![]() (16)
(16)
The equation is linearised by taking the logarithm on both sides of equation and is expressed as,
![]() (17)
(17)
where ![]() is the heavy metal amount that is adsorbed per unit mass of AABS in mg・g−1;
is the heavy metal amount that is adsorbed per unit mass of AABS in mg・g−1; ![]() is the maximum adsorption capacity in mg・g−1;
is the maximum adsorption capacity in mg・g−1; ![]() is the free energy of sorption per mole of the sorbate as it migrates to the surface of AABS from an infinite distance in the solution in mol2・kJ−2, and
is the free energy of sorption per mole of the sorbate as it migrates to the surface of AABS from an infinite distance in the solution in mol2・kJ−2, and ![]() is the Polanyi potential. The Polanyi potential
is the Polanyi potential. The Polanyi potential ![]() can be given as:
can be given as:
![]() (18)
(18)
where ![]() is the gas constant in kJ・K−1・mol−1, and
is the gas constant in kJ・K−1・mol−1, and ![]() is the absolute temperature in
is the absolute temperature in![]() . A plot of
. A plot of ![]() versus
versus ![]() gave a linear plot (not shown) from which
gave a linear plot (not shown) from which ![]() and
and ![]() are obtained from the slopes and the intercepts respectively (Table 4). Similarly, the
are obtained from the slopes and the intercepts respectively (Table 4). Similarly, the ![]() value obtained was then used to estimate the mean free energy of adsorption
value obtained was then used to estimate the mean free energy of adsorption ![]() which is calculated by,
which is calculated by,
![]() (19)
(19)
The values of ![]() were found to be in the range 0.29 - 1.58 kJ・mol−1 over the range of temperatures used in this study. Because E < 8 kJ・mol−1, it suggests that the biosorption of lead ion onto AABS is physically controlled.
were found to be in the range 0.29 - 1.58 kJ・mol−1 over the range of temperatures used in this study. Because E < 8 kJ・mol−1, it suggests that the biosorption of lead ion onto AABS is physically controlled.
3.8. Thermodynamics Studies for AABS
Thermodynamic parameters; standard free energy![]() , standard enthalpy
, standard enthalpy ![]() and standard entropy
and standard entropy![]() were calculated using the following equation:
were calculated using the following equation:
![]() (20)
(20)
![]() (21)
(21)
where ![]() is the Langmuir constant (L/mol),
is the Langmuir constant (L/mol), ![]() is the temperature in
is the temperature in ![]() and
and ![]() is the gas constant (kJ・mol−1・K−1). A plot of
is the gas constant (kJ・mol−1・K−1). A plot of ![]() against
against ![]() gave linear plot from which
gave linear plot from which ![]() and
and ![]() values were obtained from the slope and intercept respectively. From the pseudo-second-order rate constant,
values were obtained from the slope and intercept respectively. From the pseudo-second-order rate constant, ![]() (Table 3), the activation energy
(Table 3), the activation energy ![]() for the adsorption of Pb(II) onto AABS could be determined using equation
for the adsorption of Pb(II) onto AABS could be determined using equation
![]() (22)
(22)
where ![]() is the biosorption rate constant, A is the Arrhenius constant,
is the biosorption rate constant, A is the Arrhenius constant, ![]() is the activation energy (kJ・mol−1). By plotting
is the activation energy (kJ・mol−1). By plotting ![]() versus
versus![]() ,
, ![]() can be obtained from the slope of the linear plot and is presented in Table 6. The negative free energy values at various temperature as shown on Table 6 indicates that the adsorption of Pb(II) unto AABS is spontaneous and thermodynamically favoured. In addition, the range of values between 0 to −20 kJ・mol−1 further confirms that the adsorption process is a physisorption process. The positive value of
can be obtained from the slope of the linear plot and is presented in Table 6. The negative free energy values at various temperature as shown on Table 6 indicates that the adsorption of Pb(II) unto AABS is spontaneous and thermodynamically favoured. In addition, the range of values between 0 to −20 kJ・mol−1 further confirms that the adsorption process is a physisorption process. The positive value of ![]() obtained shows that the process is endothermic in nature and it range of values (2.1 - 20.9 kJ・mol−1) implies that the process is physically controlled. The values of
obtained shows that the process is endothermic in nature and it range of values (2.1 - 20.9 kJ・mol−1) implies that the process is physically controlled. The values of ![]() is positive indicating that there was randomness at the solid-liquid interface during the adsorption of the Pb(II) onto AABS.
is positive indicating that there was randomness at the solid-liquid interface during the adsorption of the Pb(II) onto AABS.
4. Conclusion
The biosorption experiment carried out using banana stalk (Musa paradisiaca) was found suitable for the adsorption of Pb(II). Considering the three modified banana stalk (AABS, BABS and RBS) used in this study, AABS was established to be the best adsorbent of Pb(II) with 96.76% metal removal. The operational parameter (Adsorbent dose, temperature, initial metal ion concentration, time and pH) had varied effects on the percentage Pb(II) removal. It was found out that Pseudo-second order kinetic model was suitable for the adsorption of
![]()
Table 6. Thermodynamic parameters for the biosorption of Pb(II) by AABS.
Pb(II). Four adsorption Isotherms (Langmuir, Freudlich, Temkin and Dubinin-Radushkevich) were tested and confirmed suitable to describe the nature of the adsorption process of Pb(II) removal from aqueous solution, but Langmuir Isotherm was the best. The thermodynamics studies showed that ![]() values were negative indicating that the process of Pb(II) adsorption onto AABS was spontaneous and the positive
values were negative indicating that the process of Pb(II) adsorption onto AABS was spontaneous and the positive ![]() and
and ![]() suggests that the biosorption process is an endothermic reaction.
suggests that the biosorption process is an endothermic reaction.
Acknowledgements
The authors acknowledge the support given by Dr. O. S. Bello of Department of Pure and Applied Chemistry, Ladoke Akintola University of Technology, Ogbomoso-Nigeria in making some laboratory facilities available for this work from his Third World Academy of Science (TWAS) Research Grant (Grant number: 11-249 RG/CHE/AF/AC_1_UNESCO FR: 3240262674).
NOTES
*Corresponding author.