Synthesis and Characterization of Struvite-k Crystals by Agar Gel ()
1. Introduction
Urolithiasis is the formation of urinary calculi, known as renal kidney stone, which is one of the serious and debilitating problems in the world [1] . The formations of stones have high supersaturation characteristics, because supersaturation is the driving force for crystal formation and growth [2] . The renal stones are the product of pathological bio mineralization in urinary system which is composed of several components and cannot be contributed to any single factor [3] . Also the chemical composition shows that, growth depends on diet, social class, age, sex of the person and on climatic and environmental conditions [4] [5] .
The urinary crystals are mostly composed of oxalates and phosphates. Among the phosphates, hydroxyapatite [6] , carbonate apatite [7] and brushite [8] are the common constituents of calciumphosphate crystals, whereas ammonium magnesium phosphate hexahydrate (NH4MgPO4∙6H2O) known as struvite and magnesium hydrogen phosphate trihydrates (MgHPO4·3H2O), i.e. newberyite, is the common constituents of magnesium phosphates crystals. They are reported in vesicle as well as renal calculi of adults [1] [9] [10] and children [11] - [13] . Of these, newberyite is a very rare crystalline component of urinary calculi [14] , while struvite is mostly found in urine [15] . Struvites are also known as infection crystals, because they are form due to urinary tract infections with ureolithic microorganisms in humans and animals [16] - [18] . A series of orthorhombic struvite analogues crystals (MM’ with M = Mg, Zn, Cd and M’ = K, Ti, NH4, Rb) were reported due to their biomedical importance [19] [20] . One of the analogous compounds of struvite is potassium magnesium phosphate hexahydrate (KMg-PO4∙6H2O), known as struvite-k crystal [21] - [23] , which has been approved (CNMMN-IMA2003) as a new inorganic phosphate mineral [24] . Struvite-k crystals are found in natural minerals as well as in animal urinary calculi [23] - [26] .
Gel method is the most versatile and simple technique for growing urinary crystals [27] [28] . In this method, gel acts as an inert and viscous medium for the growth of these crystals [29] [30] .
These searchers used different methods to grow analogous of struvite crystals. Ni-Struvite and Zn-Struvite were grown by slow evaporation method [31] [32] , by feeding a cottonseed meal and rice straw diet supplemented with MgO, Sun et al. [23] investigated struvite-k crystals in six goats. Zhang et al. [22] observed the dehydration characteristics of synthesized struvite-k at N2 atmosphere. Banks et al. [19] have grown the struvite-k crystals by gel diffusion technique and observed the needle shape crystals; however Chauhan et al. [21] have grown these crystals in silica gel by single diffusion gel techniques and observed different morphologies with changing growth parameters. Till today, the growth of struvite-k crystals in agar gel by single and double diffusion techniques have not been reported.
In the present work, the struvite-k crystals were grown in agar gel at ambient temperature and were characterized by optical microscopy, scanning electron microscopy (SEM), fourier transform infrared (FTIR) spectroscopy, X-ray diffraction (XRD), energy dispersive X-ray analysis (EDXA) and thermogravimetry analysis (TGA).
2. Experimental
The growth of struvite-k crystal was carried out by single and double diffusion techniques described by Henisch [33] . The glass test tubes of size 15 cm in length and 1.8 cm in diameter and U-tube of size 25 cm in length and 2.5 cm in diameter were used as crystallization vessels. Agar gel (Himedia) solution was prepared by mixing (0.25 to 1.0 g) of agar powder in 100 ml double distilled water at boiling temperature. Potassium dihydrogen phosphate (KDP) (Merck) of concentration (0.5 - 1.0 M) and magnesium acetate (Qualigens) of concentration (0.5 - 1.0 M) were used as reactants.
In single diffusion techniques, the agar solution was mixed with the desired concentration and appropriate volume of magnesium acetate. After setting and aging of gel, an aqueous solution of KDP was poured. Initially no precipitation was observed, however nucleations were seen in different regions of the gel within two to eight days. The neutral gel was also employed in this technique.
On reversing the reactants, one reactant KDP was poured with gel, while another reactant, magnesium acetate was poured over set gel. Initially no precipitation was observed, however single nucleation was started growing within a week. Further the growth of nuclei was resulted in the formation of single transparent prismatic type crystal.
However, in double diffusion technique, agar solution was poured in U-tube up to appropriate height and kept for setting. After setting and aging of gel, an aqueous solution of KDP was poured in one limb, while the magnesium acetate in other limb. Both the reactants were diffused slowly through the gel column. Nucleations were starts to grow at the middle of U tube. Growth process took eighty days to complete. The grown crystals were harvested from the gel and washed by double distilled water. Further, grown crystals were characterized by, Optical Microscopy, SEM, FTIR, XRD, EDXA and TGA.
3. Result and Discussion
In single diffusion technique, while growing the struvite-k crystals using silica gel, according to Chauhan et al. [21] , the formation of Liesegang rings were observed. It was also reported that, the number of rings depends on pH of gel. However in the present study, while growing the struvite-k crystals in agar gel, no Liesegang rings, but heavy nucleation was observed in both single and double diffusion technique. To control the nucleation, different growth parameters such as, concentration of gel, concentration of reactant, aging of gel and volume of reactants was changed, which can be seen in Figure 1 and the optimum condition was established shown in Table 1. Figure 2 shows some transparent dendrites, star, platy, prismatic, and polycrystalline types of crystals grown in single diffusion.
Figure 3(a), shows the growth of struvite-k crystal using magnesium acetate as first reactant, (b) using magnesium acetate as a supernatant and (c) using neutral gel in magnesium acetate as first reactant by single diffusion. Figure 3(a) shows the growth of large numbers of crystals from liquid-gel interface to deep in gel. While in test tube (b), on reversing the reactant, a large nuclei is observed than those observed in test tube (a). This may be due to enough nutrient reached to the nuclei in test tube (b) while in test tube (a) nutrients has been divided, therefore they donot grow in large size. Figure 3(b) shows a big size transparent prismatic type crystal. However Figure 3(c) shows the growth of struvite-k crystals using neutral gel. So many investigators used neutral gel to grow the crystals. According to Bhavsar [34] , neutral gel reduce the rate of reaction and control the nucleation, while Patil et al. [35] reported that, the neutral gel increase the size of crystals. However Dalal et al. [36] found that the neutral gel reduced the number of nucleation, but could not change the size of crystals. In the present study while using the neutral gel, it was observed that, the large numbers of nuclei were found to be grown near the gel-gel diffusion interface. Due to heavy nucleation, the size of the grown crystals was not increased as shown in Figure 3(c).
In double diffusion, the crystals were grown at the middle of U tube as shown in Figure 4. The obtained optimum condition for double diffusion was reported in Table 1. While growing the crystals in double diffusion technique, it was found that, the shape and surface morphology of the grown crystals was changed. Dumb-bell shape morphology was observed in double diffusion as shown in Figure 5. Variations in morphology were due to changes in the kind of diffusions of the solvents [37] .
![]()
Figure 1. Struvite-k crystals with controlled nucleation in single diffusion.
![]()
Table 1. Optimum condition for the growth of struvite-k crystals.
![]()
![]()
![]()
Figure 2. Struvite-k crystals in single diffusion. (a) Transparent star type; (b) Platy and prismatic type; (c) Polycrystalline type.
![]()
Figure 3. Growth of struvite-k crystal in single diffusion.
![]()
Figure 4. Growth of struvite-k crystal in double diffusion.
![]()
Figure 5. Opaque dumb-bell shape struvite-k crystals grown in double diffusion.
3.1. Characterization
3.1.1. Optical Microscopy
The microscopic photographs of struvite-k crystals shown in Figure 6 were observed under a CZM4 LAB- OMED stereo microscope. The morphology of the crystal is the result of the relative growth rates of its various faces. The crystal surfaces help to understand the mechanism and nature of crystals growth [38] . Figure 6(a), shows a semi-transparent crystal, with rough surface made up of peats and elongated as a rod in one side while a tip in other. However in Figure 6(b) an oval shaped crystal was observed, it’s both the curvature are constant and the tips are in equal shape. The crystal is semi-transparent in multiple stepped pyramidal terminations and has multiple parallel prismatic faces on its upper surface.
In Figure 6(c), crystal was dendratic form and observed in triangular pyramidal edge shape. The center of the crystal was cloudy whereas the granular dendratic patterns were transparent. The shape of such crystal was due to entire composition of intergrown crystals.
Figure 6(d), shows the morphology of struvite-k crystals in double diffusion techniques. The grown crystal was in dumbbell shape having rounded ends and numbers of elliptical steps. This growth was due to the layer growth. The crystal layer ended on a rough surface composed of crystals with their points up and gave birth to a new layer of crystals arranged in a similar manner [39] . In double diffusion, the change in size and habits of the crystals were affected by the numerous parameters relating to reaction, viscosity of gel, diffusion of reactants and the solvent employed in gel [40] .
3.1.2. Scanning Electron Microscopy
The morphology of struvite-k crystals was observed by using SEM as shown in Figures 7(a)-(c).
Figure 7(a) shows that the crystal was formed in elongated rectangular bar shaped morphology having with rough surface in an average particle size is 11.0 × 2.0 μm. Both the ends of this micro crystal are flat. In Figure 7(b), the particle was observed in needle shape with flat surfaces. The length of this needle shaped micro crystal was approximately 8.8 μm. However the layer to layer morphology with an average size of 78.9 × 39.0 × 47.0 μm was observed in Figure 7(c).These morphologies may be due to the fact that, the microcrystals were not growing in lateral direction, but also in particular thickness.
3.1.3. FTIR Spectroscopic Analysis
FTIR spectrum of the synthesized struvite-k crystal was recorded in the range of 400 to 4000 cm−1 is presented in Figure 8 and assigned vibrational band with observed wave numbers are summarized in Table 2.
The two broad IR bands located at 3514.42 cm−1 and 3275.24 cm−1 [21] are assigned to the H-O-H stretching vibrations of the water in crystals, while the two weak bands at 2478.61 cm−1 and 2393.74 cm−1 correspond to H-O-H stretching vibrations of cluster of water molecules in crystallization [22] . The medium intense bands appeared at 1699.34 cm−1and 1653.05 cm−1 indicate the H-O-H bending modes of vibrations [41] [42] , while a medium absorption band at 891.14 cm−1 indicates the wagging modes of vibration of the coordinated water and metal-oxygen bond in the complex [21] .
In ideal case, free phosphate ion exhibits four fundamental modes of vibrations at 1082 (ν3), 908 (ν1), 515 (ν4) and 363 (ν2) cm−1 [32] . Of these ν1 and ν3 are stretching, ν2 and ν4 are bending vibrations. In between these four
![]()
Figure 6. Stereoscope microscopy morphologies of struvite-k crystals. (a) Semitransparent with fine tip; (b) Translucent oval shape; (c) Milky star shaped; (d) Dumb-bell shaped crystals.
![]()
Figure 8. FTIR spectrum of struvite-k crystal.
![]()
Table 2. FTIR wave numbers and vibrations assignment of struvite-k crystals.
vibration modes only, ν3 and ν4 are infrared active [43] . In general, most of the phosphate ions are distorted from the ideal tetrahedral symmetry, but allow the non-active vibrations ν1 and ν2 to absorb energy in the infrared region [32] . In the present study, the band observed at 1022.31 cm−1 is attributed to ν1 symmetric stretching vibrations, whereas the bands at 1062.81 cm−1, 1165.04 cm−1 and 1232.55 cm−1 are due to the ν3 asymmetric stretching vibrations of PO4 units in struvite-k crystals which correspond to previous reported values [21] [42] [44] - [46] . However, the peaks near the region 500 cm−1 correspond to the ν2 symmetric bending vibrations and ν4 asymmetric bending vibrations of PO4 units. A shoulder at 684.75 cm−1 indicates the presence of metal-oxygen bond [22] [41] .
Thus, FTIR spectroscopy confirmed the growth of struvite-k crystals was due to the presence of water molecules, stretching and bending vibrations of phosphate (PO4) ions and Mg-O bond.
3.1.4. X-Ray Analysis
The XRD pattern of struvite-k crystal is shown in Figure 9. The crystalline phases and d-values obtained from the XRD have been compared with the JCPDS data. The XRD pattern of Figure 9 matches with the JCPDS files [47] - [49] , indicating that the sample consisted of struvite-k crystalline.
The grain size is determined by measuring the width of the line with highest intensity peaks. The average grain size has been obtained from the XRD pattern using the Scherrer’s formula [50] , Grain size D = 0.94λ/β∙cosθ. Where β is the full width at half maximum (FWHM) and λ is the wave length of the X-rays. The obtained average grain size value is 87.3 nm.
Table 3 gives the position, d-values and matching peaks of the struvite-k crystals and Figure 10 shows plot identified phases of struvite-k crystal.
3.1.5. Energy Dispersive X-Ray Analysis (EDXA)
The elemental composition of the sample is identified using energy dispersive X-ray analysis. The EDX spectrum of struvite-k crystal is shown in Figure 11. The higher peaks show the concentrated element as Mg, P and O in the specimen. Table 4 shows the EDX data of struvite-k crystals.
3.1.6. Thermogravimetry (TGA)
Chauhan et al. [21] and Siyu Zhang et al. [22] reported the dehydration characteristics of struvite-k crystals by TGA. In the present study, grown sample was also analyzed by TGA for thermal studies.
Figure 12 shows the thermogram of struvite-k crystals recorded in the temperature range 50˚C - 700˚C at the rate of 10˚C/min. The curve indicates that the mass loss is in one step i.e. dehydration of crystals and being started from 90˚C and end at 600˚C. Up to this temperature, the mass of sample was found to be 63.11% of its original mass and then it remains stable up to 700˚C. This continuous loss of mass observed in TGA, corresponds to the dehydration of the struvite-k crystal. On the basis of TGA studies, the following tentative mechanism has been expected during the complete dehydration of struvite-k crystals.
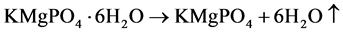
The simultaneous loss of water molecules from the struvite-k structure may be taken as a function of temperature rather than in distinct steps [22] . In TGA study, the total mass loss is found to be 36.89%, which is due to the loss of water in crystallization. Thus TGA study confirmed the structure of struvite-k crystals.
4. Conclusions
The struvite-k crystals are found to be grown by single and double diffusion techniques using agar gel as a medium of growth. Optical microscopy and SEM analysis show the growth mechanism and morphological properties of struvite-k crystals, such as star, platy, rectangular and prismatic obtained in single diffusion, while
![]()
Figure 9. XRD pattern of Struvite-k crystal.
![]()
Table 3. X-ray diffraction analysis of struvite-k crystals.
![]()
Figure 10. Plot of identified phases of struvite-k crystal.
![]()
Figure 11. EDX spectrum of struvite-k crystal.
![]()
Table 4. EDX data of struvite-k crystals.
dumbbell shape in double diffusion. FTIR spectroscopy reveals the various functional groups present in crystal. An X-ray diffraction pattern confirms the crystalline nature in the average grain size of 87.3 nm. EDXA leads to the conclusion that crystal shows the presence of magnesium, phosphor, carbon and oxygen. Whereas TGA study reveals that the one stage dehydration is due to loss of water. The proposed chemical formula and crystal structure for the grown sample is in good agreement with the results obtained from TGA studies.
Acknowledgements
The authors are thankful to The Principal, Shri V. S. Naik Arts, Commerce and Science College, Raver (M.S.), to the Director, UDCT, NMU, Jalgaon and to the Director, SICART (Gujarat) for providing experimental facilities.
NOTES
*Corresponding author.