Kinetic and Thermodynamic Study for Fenton-Like Oxidation of Amaranth Red Dye ()
1. Introduction
The need of industry for dye has shown a high pollutant potential, specially the use of azodyes [1] , for example, tartrazine [2] , amaranth [3] and others. Amaranth is used as food dye; cosmetic dye and can applied for natural and synthetic fibers, leather, paper, phenol formaldehyde resins [4] . The FDA in the United States has banned amaranth and it is also banned in Russia, Norway and Austria [5] . Owing to their toxicity, many studies have been done to remove them from water source. Biological (biodegradation) [6] and chemical methods (chlorination, ozonation) [7] are the most frequently used methods for removal of dyes from effluent water streams. But, these traditional processes for treatment of the effluents prove to be insufficient to purify waste water after the different operations of waste waters dyeing and washing.
Advanced oxidation processes (AOPs) are alternative methods for the complete degradation of dye. The usage of the advanced oxidation processes (AOPs) have improved during the last decade since they are able to eliminate the problem of dye destruction in aqueous systems. AOPs were based on the generation of very reactive species such as hydroxyl radicals (•OH) that oxidize a broad range of pollutants quickly and non-selectively. AOPs such as Fenton and Photo-Fenton catalytic reactions [8] [9] , H2O2/UV processes [9] .
The aim of this work was to find out the potential of Fenton-like for the decolorization of amaranth as model azo dye and examine the effect of the major system parameters on the decolorization kinetics of amaranth dye. Such parameters are the pH, concentration of ferric chloride, H2O2, dye and temperature of ambient. Also the study gave attention to the effect some inorganic electrolytes.
2. Experimental
2.1. Reagents and Materials
All chemicals were of pure grade and were used without further purification. Amaranth Red purchased from Fisher Scientific company chemical manufacturing division Fair town, New Jersey (molecular weight = 604.47, lmax = 520 nm). The chemical structure and uv-vis spectrum of amaranth red dye is given in Figure 1. FeCl3, NaCl, NaNO3 and Na2CO3 were purchased from Merck. Hydrogen peroxide solution (35%) was of analytical grade. All solutions were prepared using bidistilled water. Stock solutions of dye (1 mM), FeCl3 (10mM) were prepared in 0.01 M of HCl. All experiments were performed at pH below 3.
2.2. Kinetic Experiments
The kinetic measurements were carried out spectrophotometrically using 292 Cecil spectrophotometer was equipped with constant temperature cell holder attached to thermostatic controlled bath with temperature stability of ±0.1˚C. The reactants were thermostated for 15 min, then mixed thoroughly and quickly transferred to an absorption cell. The progress of the reaction was monitored at 520 nm. The pH of the reaction was adjusted using Griffin pH-meter fitted with a combined glass calomel electrode.
3. Results and Discussion
The present study concerns with the oxidative decolorization of amaranth by Fenton-like reaction. The absorbance of amaranth at λmax = 520 nm decreased with time as shown in Figure 2. The kinetics measurements were carried out under pseudo first order conditions, where hydrogen peroxide concentration was in excess than that of dye. The plot of logarithmic absorbance vs. time was linear. This indicates the pseudo first-order kinetics of the reaction with respect to the Amaranth concentration.
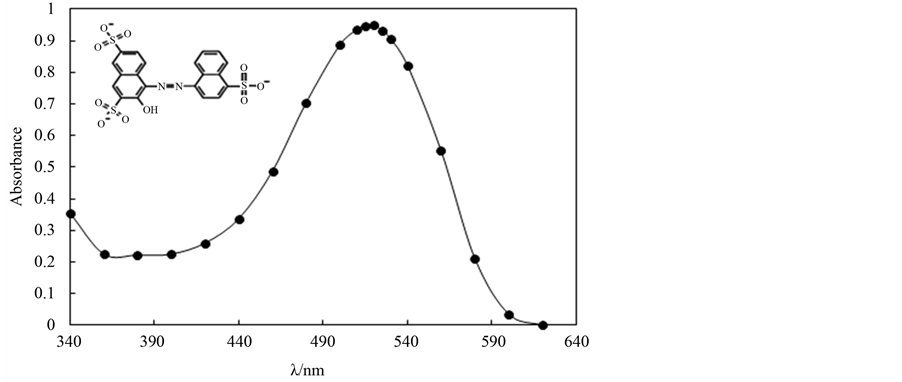
Figure 1. Chemical structure, uv-vis spectrum of amaranth red dye. [dye] = 5 × 10−5 mol∙dm−3.

Figure 2. The change in absorbance of amaranth vs time at l = 520 nm. [dye] = 5 × 10−5 mol∙dm−3, [H2O2] = 5 × 10−3 mol∙dm−3, [Fe3+] = 5 × 10−4 mol∙dm−3 and pH = 2.6.
3.1. Effect of pH
Advanced oxidation processes are powerful alternative methods of wastewater treatment. This method based on the production of powerful oxidant, HO. (E = 2.8 V versus NHE). These radicals are capable to degrade recalcitrant organic compounds under mild experimental conditions [10] . The general mechanism of Fenton reaction [11] is
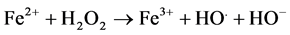 (1)
(1)
Then, Fe3+ ions can be reduced by excess H2O2 to form Fe2+ ions and more HO. radicals. Second reaction is called Fenton-like [12] allowing Fe2+ regeneration leading to catalytic mechanism (reactions, 2 - 6),
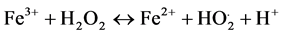 (2)
(2)
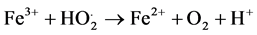 (3)
(3)
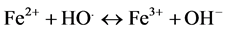 (4)
(4)
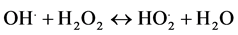 (5)
(5)
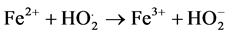 (6)
(6)
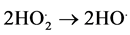 (7)
(7)
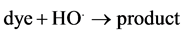 (8)
(8)
As clear from above mechanism the amount of HO. depends on the pH of solution. The effect of pH was studied in pH range of 2 - 3. The results showed that increasing pH from 2 to 2.6 ± 0.1 increases the observed rate constant, kobs, from 0.45 × 10−3 s−1 to 8.9 × 10−3 s−1 (Figure 3). The drastic decrease in the rate of decolorization at pH = 2 is attributed to drastic reduction of hydroxyl radicals resulted from the stabilization of hydrogen peroxide. Also Figure 3 showed that increasing pH above 2.6 decreases the observed rate constant. Therefore, the optimal pH value for the decolorization of amaranth is 2.6. This is in agreement with earlier results [8] [11] -[15] . These studies showed that there is optimum pH value (pH = 3).
3.2. Effect of Ferric Chloride Concentration
No reaction was observed between amaranth and hydrogen peroxide in absence of ferric ions. Ferric ion concentration shows acceleration effect on the decolorization rate of amaranth. Keeping temperature at 35˚C, [dye] = 5 ´ 10−5 mol∙dm−3 and [H2O2] = 5 ´ 10−3 mol∙dm−3 using different concentrations of ferric chloride namely, 1 - 3.75 ´ 10−4 mol∙dm−3, the decolorization rate increased by increasing ferric ions concentrations. This is attributed to increase HO. concentrations. Above that concentration the rate decreased, Figure 4. This could be at-
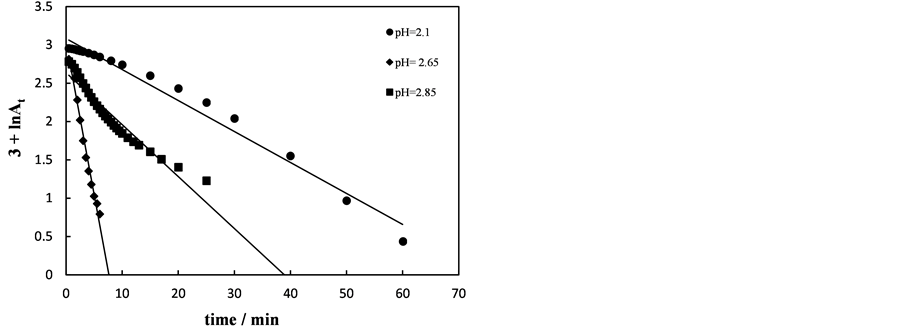
Figure 3. First order plots for decolorization of amaranth at various pH. [dye] = 5 × 10−5 mol∙dm−3, [H2O2] = 5 × 10−3 mol∙dm−3, [Fe3+] = 5 × 10−4 mol∙dm−3 and T= 35˚C.
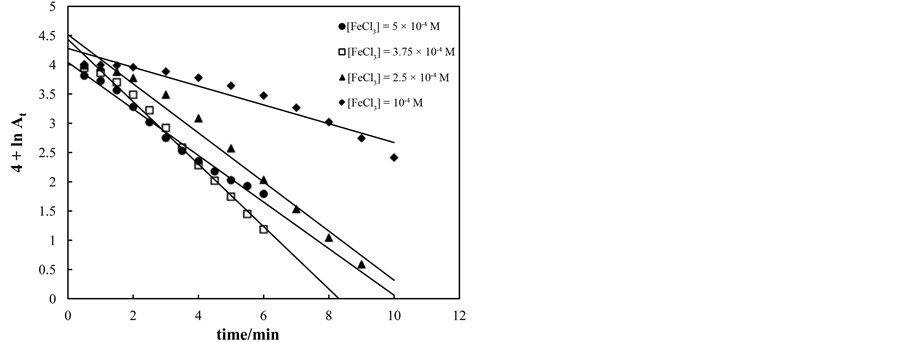
Figure 4. First order plots for decolorization of amaranth at various Fe3+ concentrations. [dye] = 5 × 10−5 mol∙dm−3, [H2O2] = 5 × 10−3 mol∙dm−3, pH = 2.65 and T = 35˚C.
tributed to the increasing in [Fe3+] increases [Fe2+] which scavenging the HO. [8] [15] . Maximum rate at [Fe3+] = 3.75 ´ 10−4 mol×dm−3, almost, 95% color removed was observed. Khataee et al. [16] reported that increasing the concentration of Fe3+ ions increases the decolorization rate of brilliant blue and maximum rate was at [Fe3+] = 2 ´ 10−4 mol∙dm−3.
3.3. Effect of H2O2 Concentration
The effect of hydrogen peroxide concentration on the rate of decolorization of amaranth studied at pH equals 2, [dye] = 5 ´ 10−5 mol×dm−3 and [Fe3+] = 5 ´ 10−4 mol×dm−3. Figure 5 showed that the decrease in dye concentration as function of time was dependent of hydrogen peroxide concentration. Increases of hydrogen peroxide concentrations in range of 5 - 25 ´ 10−3 mol∙dm−3 increases the rate constant of decolorization from 0.5 ´ 10−3 to 2 ´ 10−3 s−1. This is due to the increase of HO. radicals [8] [15] . Plot of lnkobs versus ln[H2O2] yields straight line with slope of unity indicates the reaction is first order in hydrogen peroxide.
3.4. Effect of Amaranth Concentration
The effect of initial dye concentration of aqueous solution of amaranth on Fenton-like process was investigated since pollutant concentration is important parameter in wastewater treatment. Increasing the initial amaranth concentration from 2 to 4 × 10−5 mol∙dm−3 did not show any effect on the decolorization rate, Figure 6. Also
Figure 5. First order plots for decolorization of amaranth at various H2O2 concentrations. [dye] = 5 × 10−5 mol∙dm−3, [Fe3+] = 5 × 10−4 mol∙dm−3, T = 35˚C and pH = 2.
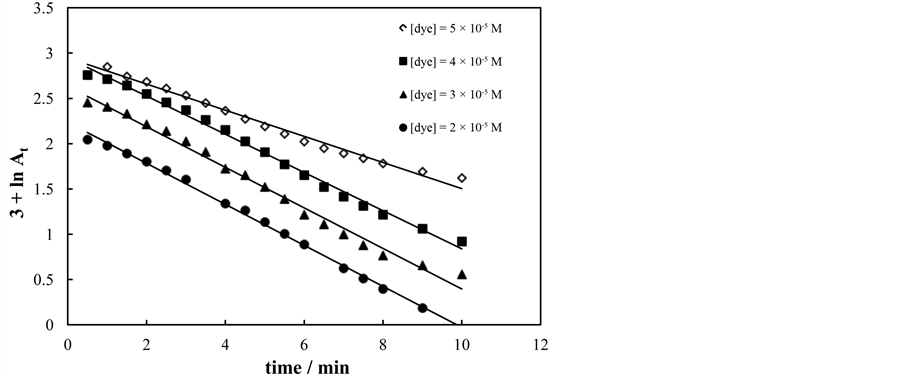
Figure 6. First order plots for decolorization of amaranth at various dye concentrations. [H2O2] = 5 × 10−3 mol∙dm−3, [Fe3+] = 5 × 10−4 mol∙dm−3, T = 30˚C and pH = 2.65.
Figure 6 shows that increasing the concentration of dye above (4 × 10−5 mol∙dm−3) decrease the rate of decolorization. This attributed to relatively lower of HO. results from the increasing of amaranth concentration while concentration of H2O2 and Fe3+ remains the same. The obtained results were in good agreement with earlier reported [11] [13] -[15] .
3.5. Effect of Temperature
The variation of the temperature in range of 298 - 308 K increases the rate of decolorization of amaranth. No optimal temperature in this study was detected as opposed to the literature reports [17] [18] in which 30˚C are stated as optimal temperature for Fenton oxidation. Another optimal temperature, 50˚C was reported on decolorization of some dyes by Fenton-like reaction [8] . The activation energy was calculated from Arrhenius plot and Eyring equation and was found to be 104.79 kJ∙mol−1. The other thermodynamic parameters are given in Table1 As observed from Table 1 positive value of ΔS* indicates reaction is favor. The ΔH* positive value indicates that the process is endothermic.
3.6. Effect of Inorganic Anion Concentration
Inorganic anions occur naturally in waste water (e.g.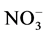 ) or may be added to facilitate the dyeing (e.g. Cl− and
) or may be added to facilitate the dyeing (e.g. Cl− and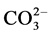 ). The presence of inorganic anions in textile wastewaters plays an important role in the oxidation kinetics of different dyes. Inorganic anions may induce or reduce the rate of oxidation.
). The presence of inorganic anions in textile wastewaters plays an important role in the oxidation kinetics of different dyes. Inorganic anions may induce or reduce the rate of oxidation.
3.6.1. Influence of NaNO3 Concentration
Decolorization rate of amaranth did not affect by addition of NaNO3 (Table 2).
3.6.2. Influence of Na2CO3 Concentration
Different concentrations of Na2CO3 were used to study the effect of carbonate ions on the oxidation of amaranth. Carbonate ions were present mainly as H2CO3, since the experiments were performed at pH ≤ 3. Presence of bicarbonate ions in the course of oxidation may decrease the decolorization rate due to scavenging of OH• by 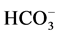
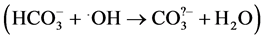 . Production of
. Production of 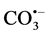 which is less reactive than hydroxyl radical [19] lowered the levels of .OH during the course of the reaction hence decreasing the decolorization rate. It was observed that the decolorization rate constant (5.295 × 10−3 s−1) in the absence of carbonate ions decreased to 1.74 × 10−3 s−1 due to the presence of 8 × 10−3 mol∙dm−3 Na2CO3, Table2
which is less reactive than hydroxyl radical [19] lowered the levels of .OH during the course of the reaction hence decreasing the decolorization rate. It was observed that the decolorization rate constant (5.295 × 10−3 s−1) in the absence of carbonate ions decreased to 1.74 × 10−3 s−1 due to the presence of 8 × 10−3 mol∙dm−3 Na2CO3, Table2
3.6.3. Influence of NaCl Concentration
Addition of 0.085 mol∙dm−3 of NaCl decreased rate constant from 5.32 × 10−3 s−1 (in absence of chloride) to 1.6 × 10−3 s−1. The inhibitive effect of chloride can be explained by scavenging effect of chloride ion on .OH (Equation (9)).
 (9)
(9)
Increasing the concentration of chloride up to 0.340 mol∙dm−3 had no significant effect (Table 2). The obtained results were in good agreement with earlier reported [8] [11] [14] .

Table 1. Thermodynamic parameters for decolorization of Amaranth by Fenton-like reagent.

Table 2. Observed first order rate constants for the decolorization of Amaranth by Fenton-like reagent at temperature = 35˚C at different concentration of inorganic anions.
4. Conclusion
The decolorization kinetics of amaranth red in aqueous solution was studied using Fenton like reaction in dark environment. The results showed that the Fenton-like is powerful method for decolorization of amaranth red dye. The reaction was first order in dye and H2O2. The Fenton-like oxidation of amaranth red dye showed maximum rate at pH = 2.6 and [Fe3+] = 3.75 × 10−4 mol∙dm−3.