ACE-I inhibitory peptide fractions from enzymatic hydrolysates of velvet bean (Mucuna pruriens) ()
1. INTRODUCTION
The nutritional and functional properties of food proteins have been investigated for many years. The nutritional quality of a protein depends on its amino acid content and on the physiological utilization of specific amino acids after digestion and absorption. In terms of their functional properties, proteins contribute to the physicochemical and sensory properties of various protein-rich foods. Furthermore, the possibility to release biologically active peptides from food proteins has gained a lot of interest. Mucuna pruriens is an underutilized tropical legume grown in Africa, South America and Asia as a green manure/cover crop. It is rich in protein (23% - 35%) and has good potential as a cheap and alternate source of protein [1]. Bioactive peptides are inactive within the sequence of the parent protein and can be released by proteolytic enzymes during gastrointestinal digestion or during food processing. Once they are liberated in the body, bioactive peptides can affect numerous physiological functions of the organism [2].
Among the different classes of bioactive peptides, the antihypertensive peptides are the best known. The main group of the antihypertensive peptides corresponds to the inhibitors of Angiotensin Converting Enzyme (ACE) [2]. ACE is a membrane-bound exopeptidase and is localized on the plasma membranes of various cell types, including vascular endothelial cells, microvillar brush border epithelial cells (e.g., renal proximal tubule cells), and neuroepithelial cells. It is this membrane-bound ACE that is thought to be physiologically important. ACE also exists in a soluble form in plasma, but this form may simply reflect turnover and clearance of membrane-bound ACE. ACE (also known as kininase II) converts the inactive decapeptide, angiotensin I, into a potent vasoconstrictor, the octapeptide angiotensin II and metabolizes a number of other peptides, including the vasodilator peptides bradykinin and kallidin, to inactive metabolites. Thus, functionally, the enzymatic actions of ACE potentially result in increased vasoconstriction and decreased vasodilation [3].
Due to their hypotensive effect, ACE-inhibitory peptides are of special interest, as hypertension is a major risk factor for both coronary heart disease and stroke, and represents an increasing health problem in Western countries. Different medical drugs, including ACE inhibitors, antagonists of angiotensin II type 1 receptors, betablockers, calcium antagonists and diuretics, have been developed for the treatment of hypertension [4]. According to Nussberger [5], more than 40 million patients are treated worldwide with ACE-inhibiting drugs, such as Captopril or Enalapril, which are structurally related to the ACE-inhibiting peptides present in snake venom. Smaller reductions in average blood pressure may be obtained by nutritional measures, such as reducing sodium intake or increasing daily consumption of ACE-inhibitory peptides. Because of their therapeutic potential for treatment or prevention of disease, bioactive peptides with ACE inhibitory activity may be used as components in functional foods or nutraceuticals. The results of a large meta-analysis indicate that even a 2 mm Hg-lower usual SBP would involve about 10% lower stroke mortality and about 7% lower mortality from ischemic heart disease in middle age [6].
For the content mentioned above, the present study objective was to determine ACE inhibitory activity of peptide fractions obtained by enzymatic hydrolysis from Mucuna pruriens proteins.
2. MATERIALS AND METHODS
2.1. Seeds and Chemicals
Pods of Mucuna pruriens were collected in Yucatan, Mexico. After thoroughly drying, the pods were thrashed to remove seeds. The seeds, after thorough clearing and removal of broken seeds, foreign materials and immature seeds, matured and dried seeds were stored in airtight plastic jars at room temperature (25˚C). All chemicals were reagent grade or better and purchased from Sigma Chemical Co. (St. Louis, MO, USA).
2.2. Mucuna pruriens Flour
Selected grains were ground in a disk mill (model 4-E Quaker, Mill Straub Co., Philadelphia, PA, USA) and then sifted through 4.76 and 2.38 mm screens in order to remove the smallest particles before the air classification. Hulls were removed with a fluidizing airbed and the flour resulted was milled in a Cyclotec mill (Tecator, Höganas, Sweden) until passing through a 0.841 mm screen.
2.3. Protein Concentrate
The protein concentrate of Mucuna pruriens bean was obtained by wet fractionation following Betancur et al. [7], with some modifications. Briefly, 5.0 kg M. pruriens bean flour was suspended in 3% sodium bisulfite in a 1:6 (w:v) ratio, pH was adjusted to 8 using 1.0 M NaOH and the suspension left to soak under constant agitation for one hour. The suspension was then passed through a 0.177 mm screen to separate the fiber solids from the protein and starch containing liquid portion. Residual solids were washed three times with 300 mL of 3% sodium bisulfite. The suspension left to sediment for 30 min to recover the starch after separation of the solubilized protein. The pH of the protein solution was adjusted to 4.2 with 1.0 M HCl. The suspension was centrifuged at 1317 g for 20 min (Mistral 3000i, Curtin Matheson Sci., Houston, TX, USA), the precipitate was freeze-dried at −47˚C and 13 × 10−3 mbar (FreeZone 4.5, Labconco. Kansas City, Missouri, USA), pulverized and stored until required.
2.4. Proximate Composition of Mucuna pruriens Flour and Protein Concentrate
Proximate composition of the Mucuna pruriens flour and the derived protein concentrate was calculated using official AOAC procedures [8]: nitrogen (method 954.01); fat (920.39); ash (923.03); fiber (962.09) and moisture (925.09). Protein content was calculated as nitrogen x 6.25, and carbohydrate content was estimated as nitrogen-free extract (NFE).
2.5. Enzymatic Hydrolysis
The M. pruriens protein concentrate (5 g/100 mL) was hydrolyzed for 120 min with the Alcalase®-Flavourzyme® and pepsin-pancreatin sequential systems. At this respect, a predigestion with Alcalase® or pepsin for 60 min was followed by incubation with Flavourzyme® or pancreatin for the resting time, respectively. Hydrolysis was done under controlled conditions (temperature, pH and stirring) in a reaction vessel equipped with a stirrer, thermometer and pH electrode. Hydrolysis parameters with Alcalase®-Flavourzyme® were: enzyme/substrate ratio 1:10 (v/v), enzyme concentration 0.3 AU∙g−1 for Alcalase® and 50 LAPU∙g−1 for Flavourzyme®; pH 8 for Alcalase® and pH 7 for Flavourzyme®; and 50˚C temperature for both [9]. Hydrolysis parameters in the pepsin-pancreatin sequential system were: enzyme/substrate ratio, 1:10; pH 2 for pepsin and pH 7.5 for pancreatin; and 37˚C temperature [10]. In all treatments, the reaction was stopped by heating to 80˚C for 20 min, followed by centrifuging at 9880 × g for 20 min to remove the insoluble portion.
2.6. Degree of Hydrolysis
Degree of hydrolysis (DH) was calculated by determining free amino groups with o-phthaldialdehyde following the methodology described by Nielsen et al. [11]: DH = h/htot*100; where htot is the total number of peptide bonds per protein equivalent, and h is the number of hydrolyzed bonds. The htot factor is dependent on the raw material amino acid composition and was determined by reverse-phase high performance liquid chromatography (RP-HPLC). Samples (2 - 4 mg protein) were treated with 4 mL 6 mol∙equi∙L−1∙HCl, placed in hydrolysis tubes and gassed with nitrogen at 110˚C for 24 h. They were then dried in a rotavapor and suspended in 1 mol∙L−1 sodium borate buffer at pH 9.0. Amino acid derivatization was performed at 50˚C using diethyl ethoxymethylenemalonate. Amino acids were separated using HPLC with a reversed-phase column (300 × 3.9 mm, Nova Pack C18, 4 mm; Waters), and a binary gradient system with 25 mmol∙L−1 sodium acetate containing (A) 0.02 g∙L−1 sodium azide at pH 6.0, and (B) acetonitrile as solvent. Flow-rate was 0.9 mL∙min−1, and elution gradient was: time 0.0 - 3.0 min, linear gradient A:B (91:9) to A-B (86:14); time 3.0 - 13.0 min, elution with A-B (86 - 14); time 13.0 - 30.0 min, linear gradient A-B (86:14) to A-B (69:31); time 30.0 - 35.0 min, elution with A-B (69:31).
2.7. Hydrolysates Fractionation by Ultrafiltration (UF)
The hydrolysate was fractionated by UF [12], using a high performance UF cell (Model 2000, Millipore). Five fractions were prepared using four molecular weight cutoff (MWCO) membranes: 1 kDa, 3 kDa, 5 kDa and 10 kDa. Soluble fractions prepared by centrifuging (9880 × g for 20 min) were passed through the membrane starting with the largest MWCO membrane cartridge (10 kDa). Retentate and permeate were collected separately, and the retentate recirculated into the feed until maximum permeate yield was reached at this size, as indicated by a decrease in permeate flow rate. Permeate from the 10 kDa membrane was then filtered through the 5 kDa membrane with recirculation until maximum permeate yield was reached. The 5 kDa permeate was then recirculated through the 3 kDa membrane and the 3 kDa permeate through the 1 kDa membrane. This process minimized contamination of the larger molecular weight fractions with smaller molecular weight fractions, while producing enough retentates and permeates for the following analyses. The five ultrafiltered peptide fractions (UPF) were prepared and designated as >10 kDa (10 kDa retentate); 5 - 10 kDa (10 kDa permeate-5 kDa retentate); 3 - 5 kDa (5 kDa permeate-3 kDa retentate); 1 - 3 kDa (3 kDa permeate-1 kDa retentate); and <1 kDa (1 kDa permeate).
2.8. ACE-I Inhibitory Activity
ACE-I inhibitory activity in the hydrolysate and its UPF was analyzed following the method of Hayakari et al. [13] that is based on the fact that ACE-I hydrolyzes hippuryl-L-histidyl-L-leucine (HHL) yielding hippuric acid and L-histidyl-L-leucine. This method relies on the colorimetric reaction of hippuric acid with 2,4,6-trichloro-S-triazine (TT) in a 0.5 mL incubation mixture containing 40 μmol potassium phosphate buffer (pH 8.3), 300 μmol sodium chloride, 40 μmol 3% HHL in potassium phosphate buffer (pH 8.3), and 100 mU/mL ACE-I. This mixture was incubated at 37˚C/45 min and the reaction terminated by addition of TT (3% v/v) in dioxane and 3 mL 0.2 M potassium phosphate buffer (pH 8.3). After centrifuging the reaction mixture at 10,000 × g for 10 min, enzymatic activity was determined in the supernatant by measuring absorbance at 382 nm. All runs were done in triplicate. ACE-I inhibitory activity was quantified by a regression analysis of ACE-I inhibitory activity (%) versus peptide concentration, and IC50 values (i.e. the peptide concentration in mg protein/mL required to produce 50% ACE-I inhibition under the described conditions) defined and calculated as follows:
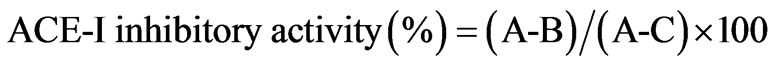
where A represents absorbance in the presence of ACE-I sample; B absorbance of the control and C absorbance of the reaction blank.
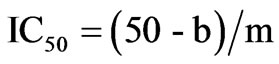 .
.
where b is the intersection and m is the slope.
2.9. Gel Filtration Chromatography of Protein Hydrolysates
After filtration through 10, 5, 3 and 1 kDa membranes in a high performance UF cell, 10 mL of the fraction with highest ACE-I inhibitory activity was injected into a Sephadex G-50 gel filtration column (3 cm × 79 cm) at a flow rate of 25 mL/h of 50 mM ammonium bicarbonate (pH 9.1). The resulting fractions were collected to assay ACE-I inhibitory activity [13]. Peptide molecular masses were determined by referring to a calibration curve running molecular mass markers on the Sephadex G-50 under identical conditions and those used for the test samples. Molecular mass standards were thyroglobulin (670 kDa), bovine gamma globulin (158 kDa), equine myoglobin (17 kDa), vitamin B12 (1.35 kDa) and Thr-Gln (0.25 kDa). Fractions selected for further peptide purification were pooled and lyophilized before RP-HPLC.
2.10. Statistical Analysis
All results were analyzed in triplicate using descriptive statistics to estimate means and variation. One-way ANOVAs were run to evaluate in vitro ACE-I inhibitory activity. All analyses were done according to Montgomery [14] and processed using the Statgraphics Plus version 5.1 Software.
3. RESULTS AND DISCUSSION
3.1 Proximate Composition of Mucuna pruriens Flour and Protein Concentrate
Proximate assay is an important criterion to assess the overall composition and nutritional status of any ingredient intended for food use. In this context, M. pruriens flour and protein concentrate were analyzed for different quality attributes such as moisture, crude protein, crude fat, crude fiber, ash and nitrogen free extract (NFE). Proximate composition showed significant variations among samples. The M. pruriensflour and protein concentrate had moisture of 7.61% and 3.26%; ash of 1.13% and 0.095%; crude fiber of 1.13% and 0.13%; fat of 5.59% and 15.22%; protein of 26.5% and 57.88% and NFE of 58.05% and 23.42%, respectively (Table 1). The low moisture content obtained in protein concentrate may afford a good keeping quality, hence, longer shelf life for concentrate.
Present findings regarding proximate composition are in conformity with values described in previous literature. Chemical composition of chickpea flour was analyzed by Aurelia et al. [15] and observed that moisture, protein, fat, fiber and ash were 9.00%, 23.08%, 6.65% and 3.21%, respectively. In another research, relative composition of moisture, protein, fat and ash of chickpea (8.01%, 22.83%, 5.43%, 3.50%, 3.04%), broad bean (12.97%, 22.61%, 2.67%, 2.46%, 2.90%) and kidney bean (9.15%, 20.09%, 2.46%, 6.78%, 3.85%) [16] showed similar results to register here. In array of investigations, variations in proximate composition of different legumes have been observed owing to different environments, genotype and analytical methods. It was further reported that protein content is sensitive to rainfall, light intensity, length of growing season, day duration, temperature and agronomic practices [17]. The proteins are polymer of amino acids and their relative proportion represents its quality that is dependent on the genetic makeup of legumes. According to Sadiq and Batool [18] variations in protein contents of different protein isolates or concentrates could possibly by due to extent of soluble proteins present in raw materials as well as the used extraction method. The protein content of M. pruriens protein concentrate (57.88%) makes from this legume a viable alternative to obtain biologically active peptides.
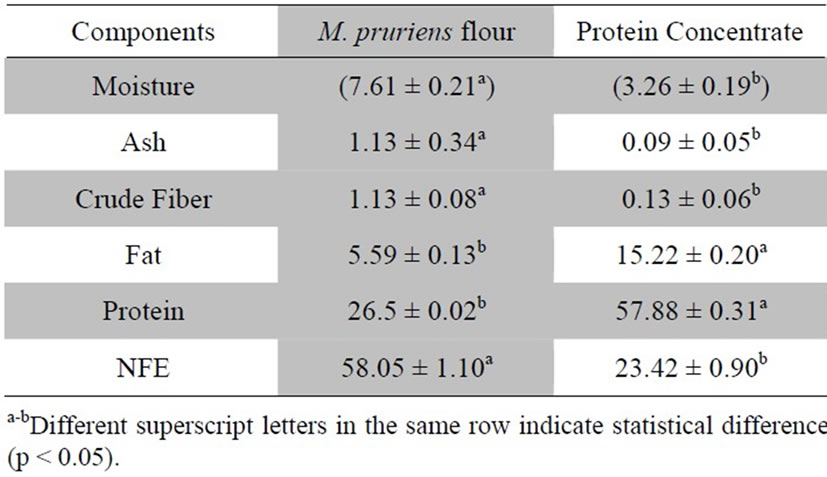
Table 1. Proximate composition of Mucuna pruriens flour and protein concentrate.
3.2 Enzymatic Hydrolysis
Alcalase®-Flavourzyme® and Pepsin-Pancreatin sequential systems were used to produce extensively hydrolyzed M. pruriens protein extracts. Degree of hydrolysis (DH) differed (p < 0.05) between the enzymatic systems with values of 29.08% and 24.78% for Alcalase-Flavourzyme Hydrolysate (AFH) and Pepsin-Pancreatin Hydrolysate PPH, respectively. The DH values were lower than those reported for hydrolysis of other legumes like Vigna unguiculata (53.1%, 58.8% and 35.7%) with Alcalase®, Flavourzyme® and pepsin-pancreatin [19] and Phaseolus vulgaris (43.1%) with Alcalase®-Flavourzyme® for 90 min [20]. Variation in DH values was probably the result of enzymatic system hydrolytic specificity and protein source. Peptides with biological activities have generally been isolated from food proteins via hydrolysis with digestive enzymes such as pepsin, pancreatin, or chymotrypsin [9]. In comparison to animal or plant derived enzymes, microbial proteases such as Alcalase®, Protamex, and Flavourzyme® have also shown excellent potential to produce highly biological and functional hydrolysates [21].
3.3. ACE Inhibitory Activity of Protein Hydrolysates and Ultrafiltered Fractions
Numerous in vitro techniques, base on either spectrophotometric or HPLC assay, have been developed for the detection of ACE inhibitory activity of bioactive peptides. In vitro ACE-inhibitory activity is generally measured by monitoring the conversion of an appropriate substrate by ACE in the presence and absence of inhibitors. For spectrophotometric assay, method of Hayakari et al. [13] is broadly used. This method is based on the hydrolysis of HHL by ACE to hippuric acid and His-Leu. For this assay, ACE inhibitory activity is measured through the absorbance of hippuric acid after the reaction of hydrolysate samples on HHL. The quantity of hippuric acid produced by ACE is measured at 228 nm using a UV-visible spectrophotometer.The M. pruriens protein hydrolysates (AFH and PPH) and their corresponding UF peptide fractions (i.e. >10, 5 - 10, 3 - 5 and <1 kDa) were analyzed to determine their ACE-I inhibitory activity. For the above mentioned, apart from, HHL, the M. pruriens hydrolysates and their corresponding ultrafiltered fractions wereemployed as substrates. Biological activity was higher in AFH (33.13%) than PPH (30%). UF of both hydrolysates produced peptide fractions with high biological activity (Figure 1). Ultrafiltration is a technique used commonly both on laboratory and commercial scale to fractionate, purifies and concentrate proteins. Although it is possible to obtain very pure protein fractions using suitable columns as in column chromatography, UF is a low cost and easy to scale-up method used widely in
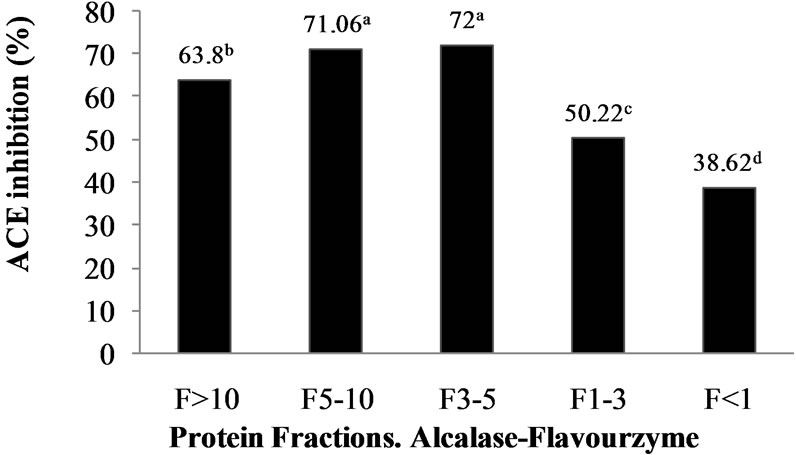
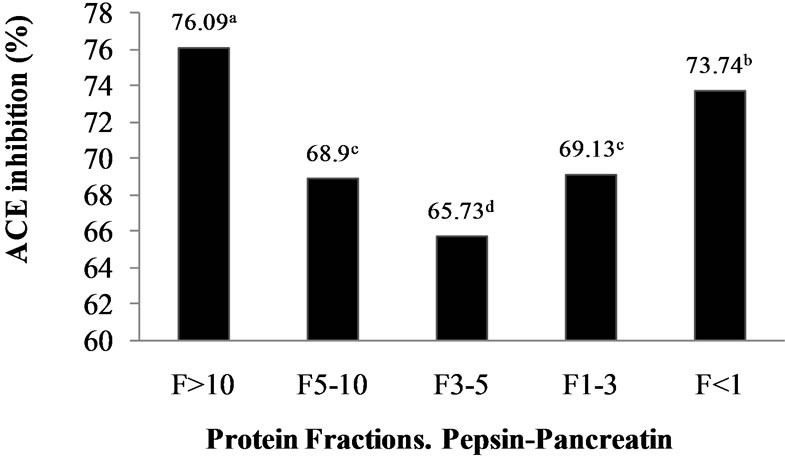
Figure 1. ACE-I inhibition (%) of peptide fractions obtained by ultrafiltration from Alcalase®-Flavourzyme® and Pepsin-Pancreatin protein hydrolysates. a-dDifferent superscript letters indicate statistical difference (p < 0.05).
food industries for quick separation and concentration of food proteins. Many researchers selected UF method in the first fractionation of ACE inhibitory peptides because a wide variety of large, medium and small peptides are generated depending on the enzyme specificity and the extent of hydrolysis during the hydrolysis reaction [22]. ACE-I inhibitory activity of peptide fractions ranged from 38.62% to 72% in the AFH and from 65.73% to 76.09% in the PPH.ACE-I inhibitory activity was significantly (p < 0.05) dependent on peptide fraction molecular weight. The results confirm that M. pruriensis a good protein source for bioactive peptide extraction by gastrointestinal or commercial proteases.
3.4. ACE Inhibitory Activity of Peptides Fractions Purified by Gel Filtration Chromatography from M. pruriens Protein Hydrolysates
In gel filtration, ACE inhibitory peptides are fractioned based on their relative size. One of the most widely used column is Sephadex for example, Sephadex G-15 for molecular weight < 1500 Da and G-25 for molecular weight < 1000 - 5000 Da. Beside, gel filtration is also used to determine the molecular weight of ACE inhibitory peptides or remove low molecular weigh impurities [22]. Gel filtration chromatography (Sephadex G-50 column) was used to generate a molecular weight profile of the M. pruriens protein hydrolysates (Figure 2). The
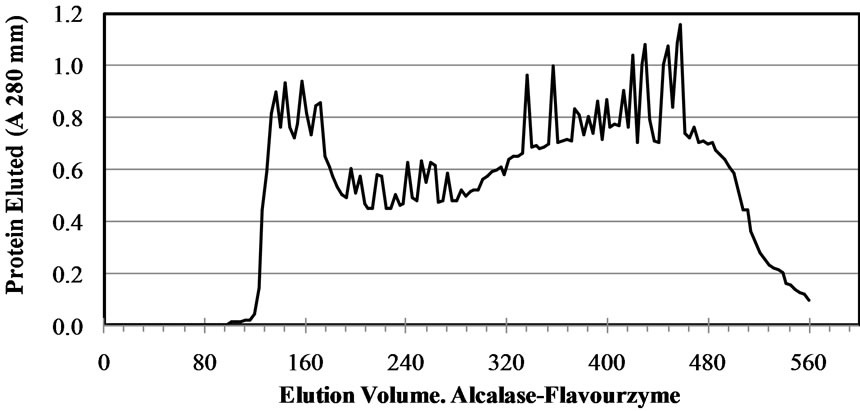
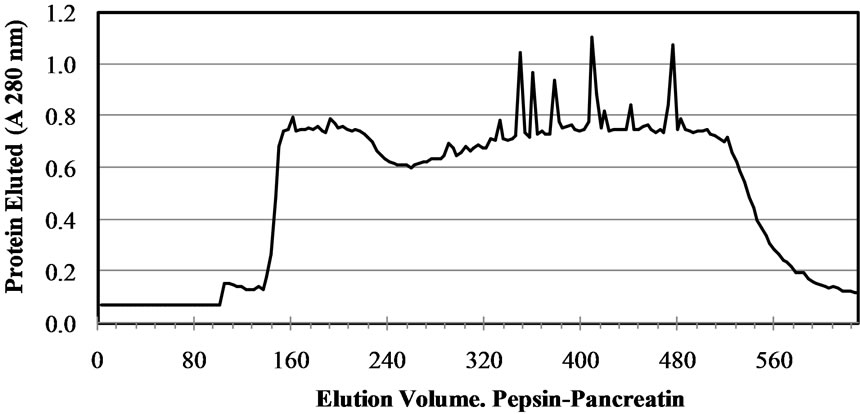
Figure 2. Elution profile of the Mucuna pruriens protein hydrolysates purified in a Sephadex G-50 gel filtration column.
profile was typical of a protein hydrolysate formed by a pool of peptides, with gradually decreasing molecular masses. Elution volumes between 406 and 518 mL included free amino acids and peptides with molecular masses ranging from 0.4 to 3.6 kDa. This range was fractionated into eleven fractions (F0 - F10) and ACE-I inhibitory activity determined for each. Fractions with elution volumes smaller than 406 mL and greater than 518 mL were not analyzed because they largely included peptides with high molecular weights, as well as free amino acids. Chain length of peptides, which are dependent on DH, is of special interest because some of the properties depend at least in part on molecular size [23]. The highest ACE-I inhibitory activity was observed in fraction F9 for both AFH (88.2%) and PPH (72%) (Figure 3). The main components of F9 in both systems were tripeptides with relative molecular weights found between 460 and 535 kDa.
4. CONCLUSION
Compared with Pepsin-Pancreatin-based M. pruriens hydrolysate, Alcalase-Flavourzyme-based M. pruries hydrolysate provided a higher degree of hydrolysis and stronger ACE-I inhibitory activity. The concentration of peptides from M. pruriens proteinhydrolysates was done using a UF system and the profile of the molecular weight distribution was analyzed by gel filtration chromatography. The ACE-I inhibitory activity of protein fractions was related with the molecular weight. The molecular weight of the most active peptide fractions was found between 460 and 535 kDa indicative of 3 amino acid residues.
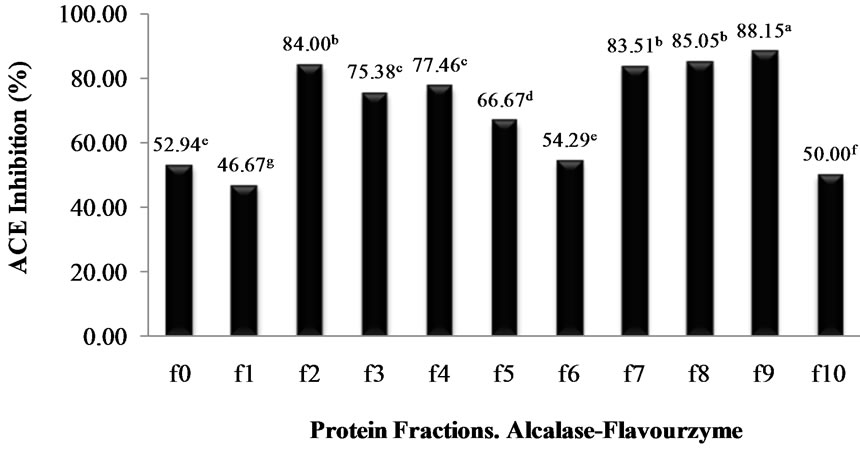
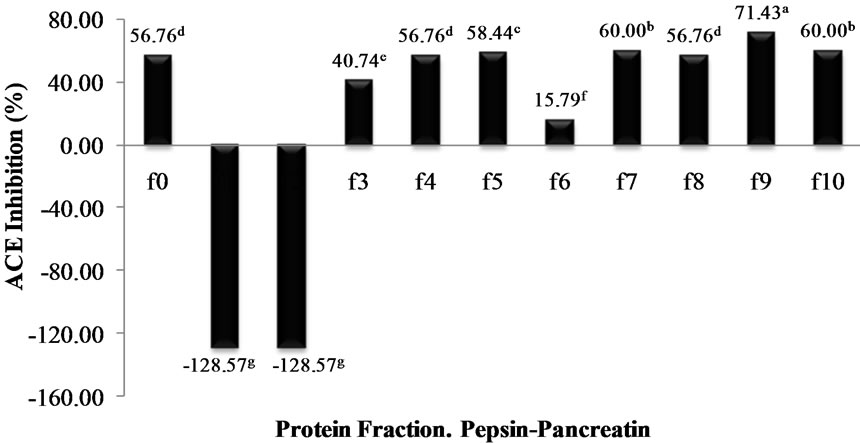
Figure 3. ACE-I inhibition percentage of the protein fractions purified by gel filtration cromatography from Alcalase®-Flavourzyme® and Pepsin-Pancreatin protein hydrolysates. a-dDifferent superscript letters indicate statistical difference (p < 0.05).
5. ACKNOWLEDGEMENTS
This research forms part of Project 154307 “Investigación científica dirigida al desarrollo de derivados proteínicos de Mucuna pruriens con potencial actividad biológica para la prevención y/o tratamiento de enfermedades crónicas asociadas al sobrepeso y la obesidad”, financed by the Consejo Nacional de Ciencia y Tecnología (CONACYT) and the Network “Bioactividad de Péptidos e Hidrolizados” supported by PROMEP-SEP, México.