Neuroscience & Medicine
Vol.4 No.1(2013), Article ID:28681,9 pages DOI:10.4236/nm.2013.41008
Scientific Interest of Social Behaviour in Animal Models of Human Diseases
![]()
1Institut of Neuroscience, Autonomous University of Barcelona, Barcelona, Spain; 2Medical Psychology Unit, Department of Psychiatry and Forensic Medicine, School of Medicine, Autonomous University of Barcelona, Barcelona, Spain.
Email: *lidia.gimenez@uab.cat
Received January 16th, 2013; revised February 20th, 2013; accepted February 28th, 2013
Keywords: Social neuroscience; Animal models; Basic and translational research; Neurodegenerative diseases; Neuropsychiatric disorders; Social behaviour
ABSTRACT
The overview shows that the scientific interest in social behaviour in mice has exponentially grown in the last two decades in parallel with advances in biotechnology and the emergence of genetically engineered mice. Most of the studies are psychopharmacological or look for the neurochemical bases of social behaviour and its alterations. However, the rol of social behaviour per se is increasing mainly in those research works aimed to model neuropsychiatric and neurodegenerative diseases. In fact, at the translational level, the study of social behaviour in murine models is relevant because changes in social behaviour are present in most neuropsychiatric and neurodegenerative disorders as well as in other diseases that, directly or indirectly, affect the sphere of social relationships. The consideration of social behaviour in the experimental design of basic and translational research works using murine models may improve the predictive validity of new preventive and/or therapeutic strategies. The present work provides conceptual description of social behaviour in mice, the tests used to measure it and analyzes its increasing interest, mostly in the area of neuroscience. It reviews the 821 scientific studies (in English) included in the MEDLINE database from 1930 to December 2012. Keywords used for the search where those related to the different kinds of social behaviour (spontaneous or induced) in mice and took into account the diversity of experimental paradigms (dyads, groups, parental relationships, isolation) and the wide spectrum of behavioural tests available.
1. Social Behaviour in Mice
Most living organisms are organized by social structures that facilitate the development of vital functions of the species such as survival (protection from predators), nutrition (collecting and providing food) and the continuity of the species itself (facilitation of the reproduction). Social structures are dynamic and when situations of inter or intra-specific conflicts appear social structures change in order to find the balance. At the clinical level, it is described that the social pattern is significantly altered in many neurological and psychiatric diseases, and this affects the development of daily routines and the quality of the interaction with their counterparts.
From basic research, animal models for neurological and psychiatric diseases try to mimic these behavioural patterns in order to provide experimental models which, with more or less validity, can be used to study the underlying biological and psychological phenomenon. Furthermore, these models provide the opportunity to assess preventive and/or therapeutic strategies. Rat was earlier considered to be the excellent animal model for scientific studies. However, recent advancement in biotechnology has made mice to emerge as the rodent specie of choice in generation of a wide range of genetic and psychopharmacological models [1]. Therefore, this paper reviews the existing literature on social behaviour in mice, including historical evolution of behavioural testings. The paper also assesses the use of behavioural tools in the study of basic traits and social behaviour in different animal models for diseases of interest.
Searches were made in MEDLINE for published studies in the English language from the beginning of the data base (1930) to December 2012 using the keywords: “mice” “social interaction”, “barbering”, “tube test”, “nesting behaviour”, “maternal separation/deprivation” “isolation”, “resident intruder test”, “sexual behaviour”, “maternal behaviour”, “social recognition test”, “olfactory discrimination test” and “playing behaviour”. The number of scientific papers found was 821.
2. Scientific Interest for the Study of Social Behaviour in Mice
Since 1930 there has been a substantial increase in both basic and translational scientific research on social behaviours in mice. The first studies basically focused on the characterization of social behaviour per se. It was not until the early 60’s that biological approaches and the use of animal (rodents) models to study varied diseases including neurological and psychiatric disorders and their impact on social relationships, were established. In the 90’s the number of scientific publications in different areas (see figure 1) and using a diversity of behavioural tests (see figure 2) experienced an exponential increase. Among the most relevant issues is the use of social interaction test [2], the resident-intruder test to measure aggression and the model of maternal deprivation mainly used to assess the ontogenetic hypothesis of schizophrenia.
3. Social Behaviour, Behavioural Tests and Modeling of Diseases
Social behaviour is a fundamental characteristic of living organisms and is defined as an interaction between members of the same specie [3]. Normal social structures implicitly involve behaviours with varying degrees of hierarchy or equality, depending on genetic factors

Figure 1. Timeline of scientific publications (1930-2012) about social behaviour in mice for areas basic and translational research. (a) Characterization of normal social behaviour; (b) Psychopharmacological studies and neurochemistry; (c) Animal models for neurological and psychiatric diseases; (d) Animal models for other non-neurological and psychiatric diseases.
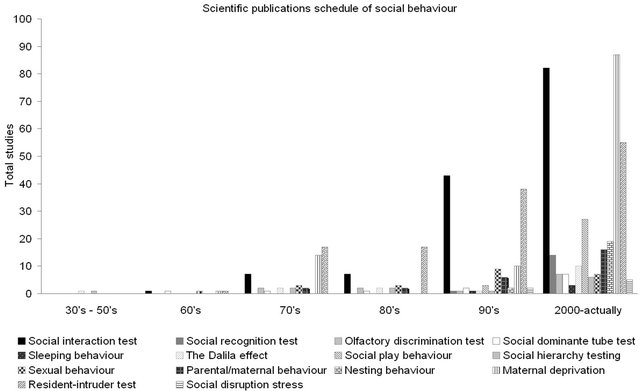
Figure 2. Timeline of scientific publications (1930-2012) about social behaviour in mice according to type test. Spontaneous social behaviour studies in dyads (social interaction test, social recognition test, olfactory discrimination test, tube-dominance test, sexual behaviour and nesting behaviour) or in group (sleeping behaviour, Dalila effect, social hierarchy test, parental/ maternal behaviour, social play behaviour) and social induced isolated behaviour studies (resident-intruder test, social disruption stress, maternal deprivation).
(mouse strain, gender, age, genetic mutations or neurochemical systems) and environmental (conditions of housing, feeding, temperature, isolation, dyads or social group membership, perinatal development.). Social behaviours, as stated before, are dynamic structures, whose deviations by default (i.e. apathy, anhedonia, isolation) or excess (i.e. aggressiveness, irritability, sexual aggression) can reach pathological range and therefore become diagnostic criteria for neurological and psychiatric diseases. In addition, alterations of social behaviours are also symptoms of other organic diseases (see Tables 1 and 2).
When studying social behaviours, several important concepts are taken into account. For instance, in any animal group each subject has social attributes that influence its social relationships with other animals. Related to this, is the concept of sociability which is defined as “the tendency to form cooperative interdependent relationships that allow two-way communication which transcends mere sexual activity” [40]. Nevertheless, the organization of a group is one of the most significant goals for many animal species, and is the basis of social organization [40]. Each species has developed patterns of behaviour and physiological mechanisms that are related to their own social organization and population dynamics [1]. It is, therefore, important to note that results obtained in behavioural paradigms in rats may be different from that obtained in mice [41]. In turn, there are also differences between mice depending on the strains a factor which is usually underestimated (the strain used is the one available rather than the one with the proper behavioural profile) [41].
It’s been told that the social structure of a group depends mainly on the dominance-subordination relationships and/or other attributes such as aggressiveness, competitiveness, individualism, etc. [40]. Thus, dominance is defined as a learned and predictable relationship established between a pair of animals (dyad) where an animal is subordinate by its partner. In this context, ranges, hierarchy and the order of dominance represent the assignment of a numerical value to an animal, in the attempt to describe the relative position of an animal in its social group [1]. On the contrary, there are social structures where agonistic behaviour is observed. These are the result of adaptive actions to solve conflicts arising between two members of the same specie through aggressive behaviour or threats, submissive or passive behaviours and playing behaviours involving physical contact [42]. Aggressive behaviour is a behaviour that causes
Table 1. Reference reviews (1930-2012) of scientific studies about social behaviour in mice. Principal test for measure social behaviour and representative literature.

harm or destruction to animals. In most animal species, males are more aggressive than females. Some authors classify the aggressiveness in aggressive competition, where two individuals within a group compete for the same resource and territorial aggression when aggression is directed to an animal that is considered has invaded a territory [40]. Based on these concepts, early studies in mice also used the social hierarchy testing (SHT) to assess each animal’s social rank within their group. That is, animals were classified in rank 1: dominant, rank 2: activesubordinated, rank 3: subordinated liabilities and rank 4, submissive [10].
In the decade of the 60’-70’s a clear scientific interest for social behaviour is observed and new methods, such as the social interaction test (SIT), are used. The test evaluate both the social and non-social behaviours and distinguish a great variety of behavioural elements such as social investigation (sniffing the anogenital region, the head, or
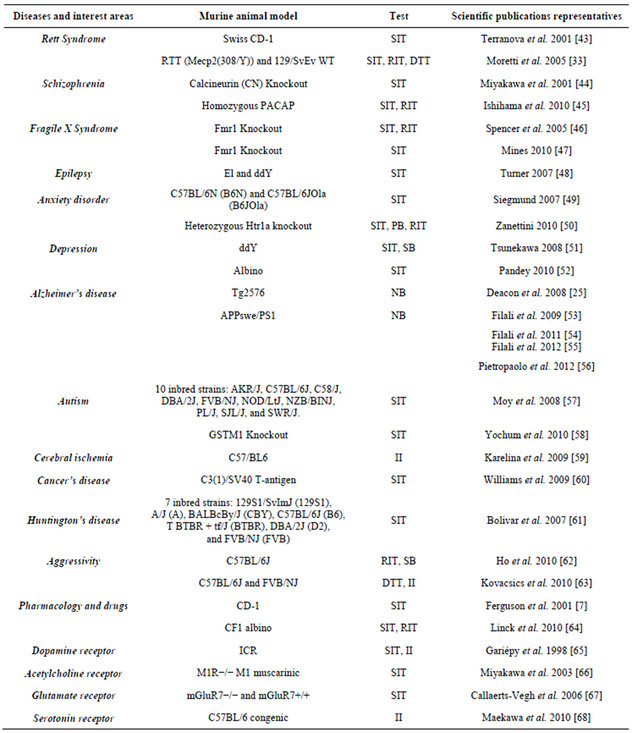
Table 2. Representative examples of the use of mouse models in studies of social behaviour and their psychological bases, neurochemistry, modeling of neurological disorder, psychiatric and other diseases.
the snout of the partner), follow (following the partner around the cage), squire (following the moving partner while maintaining a constant nose contact with its fur), push under (pushing the snout or the whole anterior part of the body under the partner’s body, and then resting), crawl over (crawling over the partner’s back, crossing it transversally from one side to the other), mutual circle (partners mutually sniffing each other’s anogenital region, while describing tight circles), vibrating tail and aggressive behaviour (including fighting accompanied by biting and blows to the head) as well as social inactivity (lying flat or standing still while maintaining close physical contact with the partner). Among non-social behaviours the SIT evaluates several actions such as exploring (moving around the cage, rearing, sniffing the air, the walls or the sawdust), digging (digging in the sawdust, pushing and kicking it around using the snout and/or both the forepaws and hind paws) and self-grooming (wiping, licking, combing, or scratching any part of own body) [6].
The component of learning and memory in social behaviour can also be studied and evaluated using two behavioural tests: the Social Recognition test (SR) in which the animal must be able to recognize the resident of a cage after having been previously exposed to its smell [9] and the Olfactory discrimination test (ODT) based on the ability of animals to discriminate two different smells (i.e. the smell of almonds versus that of lemons) [10].
Dominance relationships often have an implicit element of aggression that can be evaluated with the social dominance tube test (DTT). In this test two mice of the same genotype and gender are placed at opposite ends of an acrylic tube and released. A subject is declared a “winner” when its opponent backs out of the tube [12].
Home cage sleeping behaviour (SB) evaluates the percentage of animals sleeping huddled in the same quadrant in each cage, and it is known to be impaired in animal models of psychiatric illness such as schizophrenia [12].
In the Dalila effect or barbering, animals show shaved whiskers and hair loss which can be automatically generated by the animal itself or by a cagemate, usually the most dominant, namely the “Dalila mouse”. Scarce research is devoted to this phenomenon and it is speculated whether it is due to dominance, if it involves some level of aggression and, therefore, suffering or pain in the animal that receives it, while other authors consider that at least we can speak of a behaviour derived from social anxiety. To classify the Dalila effect the following scale is used: 0: no barbering, 1: whisker removal or shortening, 2: snout/ face denuding, 3: individual bald patches on head and body, 4: multiple alopecic areas on head and/or body; 5: severe alopecia including complete snout denuding and large pronounced alopecic areas on head and body [16].
On the other hand, there’s a group of social behaviours directly linked to reproductive functions, upbringing and ontogenetic maturation of the litters. They can be measured through a variety of successive events: mating, nesting, maternal care and games. The sexual Behaviour (SB) of the male is quantified by the latency and frequency of mounts, intromissions and ejaculations, while the female measures the level of lordosis [22]. The nesting behaviour (NB) evaluates the ability of the animal to make its nest construction [25] while the parental behaviour (PB) includes measures of protective behaviours, cleaning and food supply from mother to offspring [4]. In the social play behaviour (SPB) the elements under consideration are those of social interaction but in this case the range of age of the individuals is postnatal [39].
In other behavioural paradigms, the experimenter alters the normal conditions of housing of animals in order to induce changes or disrupt social behaviour (induced social behaviour). For instance, in the social isolation-induced (II) the animal is isolated for a month in order to increase its aggressiveness. Thereafter, the territorial aggression towards an intruder mice can be measured by the resident-intruder test (RIT). The latency of first attack, number of attacks and the time of persecution by the aggressive resident are measured [32]. A variant of this test is the Social Disruption stress (SDR) in which, using a similar procedure, the aggressive behaviour of a group of animals living in the same cage against a single attacker is being measured in a 2 minutes test [39]. In studies of ontogeny, maternal deprivation or temporary isolation rearing maternal deprivation (MD) (usually 24 hours, during the ninth postnatal day) is used to model emotional disorders and psychiatric field that allow the hypothesis on the basis ontogenetic diseases such as schizophrenia [36].
4. Conclusion
Although the first reports on social behaviour in mice were done in the 30’s, it was in the 70’s that clear scientific interest was raised. Soon after, due to the use of genetically engineered mice, interest in this field witnessed an exponential growth. Disorders in social behaviour are characteristic of many mental disorders such as autism, schizophrenia, depression and Alzheimer’s disease. These diseases have been mimicked in animal models of mice. At the moment, basic research in social behaviour is related to gender, aggression and parental relationships. The understanding of the biological and psychological basis of social behaviour is becoming increasingly relevant. Importantly, the consideration of social behaviour in the experimental design of basic and translational research works using murine models may improve the predictive validity of new preventive and/or therapeutic strategies.
5. Acknowledgements
This work was supported by Instituto de Salud Carlos III ISC3-PI10/00283.
REFERENCES
- H. J. Hedrich and G. R. Bulloch, “The Social Behaviour of Mice and Its Sensory Control,” The Handbook of Experimental Animals, The Laboratory Mice, Academic Press, London, 2004, pp. 287-298.
- S. E. File, “The Use of Social Interaction as a Method for Detecting Anxiolytic Activity of Chlordiazepoxide-Like Drugs,” Journal of Neuroscience Methods, Vol. 2, No. 3, 1980, pp. 219-238. doi:10.1016/0165-0270(80)90012-6
- M. B. Sokolowski, “Social Interactions in ‘Simple’ Model Systems,” Neuron, Vol. 65, No. 6, 2010, pp. 780-794. doi:10.1016/j.neuron.2010.03.007
- I. Branchi, I. D’Andrea, F. Gracci, D. Santucci and E. Alleva, “Birth Spacing in the Mouse Communal Nest Shapes Adult Emotional and Social Behaviour,” Physiology & Behavior, Vol. 96, No. 4-5, 2009, pp. 532-539. doi:10.1016/j.physbeh.2008.12.003
- L. Tremolizzo, M. Doueiri, E. Dong, D. R. Grayson, J. Davis and G. Pinna, “Valproate Corrects the Schizophrenia-Like Epigenetic Behavioural Modifications Induced by Methionine in Mice,” Biological Psychiatry, Vol. 57, No. 5, 2005, pp. 500-509. doi:10.1016/j.biopsych.2004.11.046
- A. Venerosi, A. Valanzano, E. Alleva and G. Calamandrei, “Prenatal Exposure to Anti-HIV Drugs: Neurobehavioural Effects of Zidovudine (AZT) + Lamivudine (3TC) Treatment in Mice,” Teratology, Vol. 63, No. 1, 2001, pp. 26-37. doi:10.1002/1096-9926(200101)63:1<26::AID-TERA1005>3.0.CO;2-G
- J. N. Ferguson, J. M. Aldag, T. R. Insel and L. J. Young, “Oxytocin in the Medial Amygdala Is Essential for Social Recognition in the Mouse,” Journal of Neuroscience, Vol. 21, No. 20, 2001, pp. 8278-8285.
- W. R. Holloway Jr. and D. H. Thor, “Social Memory Deficits in Adult Male Rats Exposed to Cadmium in Infancy,” Neurotoxicology and Teratology, Vol. 10, No. 3, 1988, pp. 193-197. doi:10.1016/0892-0362(88)90017-7
- T. Spiteri, S. Musatov, S. Ogawa, A. Ribeiro, D. W. Pfaff and A. Ågmo, “The Role of the Estrogen Receptor α in the Medial Amygdala and Ventromedial Nucleus of the Hypothalamus in Social Recognition, Anxiety and Aggression,” Behavioural Brain Research, Vol. 210, No. 2, 2010, pp. 211-220. doi:10.1016/j.bbr.2010.02.033
- R. M. Rodriguiz, R. Chu, M. G. Caron and W. C. Wetsel, “Aberrant Responses in Social Interaction of Dopamine Transporter Knockout Mice,” Behavioural Brain Research, Vol. 148, No. 1-2, 2004, pp. 185-198. doi:10.1016/S0166-4328(03)00187-6
- B. Sobottka, F. Eggert, R. Ferstl and W. MullerRuchholtz, “Changed Chemosensory Identity Following Experimental Bone Marrow Transplantation: Recognition by Another Species,” Zeitschrift für Experimentelle und Angewandte Psychologie, Vol. 36, No. 4, 1989, pp. 654- 664.
- N. Lijam, R. Paylor, M. P. McDonald, J. N. Crawley, C. X. Deng and K. Herrup, “Social Interaction and Sensorimotor Gating Abnormalities in Mice Lacking Dvl1,” Cell, Vol. 90, No. 5, 1997, pp. 895-905. doi:10.1016/S0092-8674(00)80354-2
- G. Lindzey, H. Winston and M. Manosevitz, “Social Dominance in Inbred Mouse Strains,” Nature, Vol. 191, 1961, pp. 474-476. doi:10.1038/191474a0
- E. Strozik and M. F. Festing, “Whisker Trimming in Mice,” Laboratory Animals, Vol. 15, No. 4, 1981, pp. 309-312. doi:10.1258/002367781780953040
- J. P. Garner, S. M. Weisker, B. Dufour and J. A. Mench, “Barbering (fur and Whisker Trimming) by Laboratory Mice as a Model of Human Trichotillomania and Obsessive-Compulsive Spectrum Disorders,” Comparative Medicine, Vol. 54, No. 2, 2004, pp. 216-224.
- A. V. Kalueff, A. Minasyan, T. Keisala, Z. H. Shah and P. Tuohimaa, “Hair Barbering in Mice: Implications for Neurobehavioural Research,” Behavioural Processes, Vol. 71, No. 1, 2006, pp. 8-15. doi:10.1016/j.beproc.2005.09.004
- S. Y. Long, “Hair-Nibbling and Whisker-Trimming as Indicators of Social Hierarchy in Mice,” Animal Behaviour, Vol. 20, No. 1, 1972, pp. 10-12. doi:10.1016/S0003-3472(72)80167-2
- H. K. Caldwell, O. E. Dike, E. L. Stevenson, K. Storck and W. S. Young III, “Social Dominance in Male Vasopressin 1b Receptor Knockout Mice,” Hormones and Behavior, Vol. 58, No. 2, 2010, pp. 257-263. doi:10.1016/j.yhbeh.2010.03.008
- J. Uhrich, “The Social Hierarchy in Albino Mice,” Journal of Comparative Psychology, Vol. 25, No. 2, 1938, pp. 373-413. doi:10.1037/h0056350
- T. E. McGill, “Sexual Behaviour in Three Inbred Strains of Mice,” Behaviour, Vol. 19, No. 4, 1962, pp. 341-350. doi:10.1163/156853962X00087
- D. W. Mosig and D. A. Dewsbury, “Studies of the Copulatory Behaviour of House Mice (Mus musculus),” Behavioral Biology, Vol. 16, No. 4, 1976, pp. 463-473. doi:10.1016/S0091-6773(76)91635-7
- E. F. Rissman, A. H. Early, J. A. Taylor, K. S. Korach and D. B. Lubahn, “Estrogen Receptors Are Essential for Female Sexual Receptivity,” Endocrinology, Vol. 138, No. 1, 1997, pp. 507-510. doi:10.1210/en.138.1.507
- C. Cohen-Salmon, M. Carlier, P. Roubertoux, J. Jouhaneau, C. Semal and M. Paillette, “Differences in Patterns of Pup Care in Mice V—Pup Ultrasonic Emissions and Pup Care Behaviour,” Physiology & Behavior, Vol. 35, No. 2, 1985, pp. 167-174. doi:10.1016/0031-9384(85)90331-2
- K. Nishimori, L. J. Young, Q. Guo, Z. Wang, T. R. Insel and M. M. Matzuk, “Oxytocin Is Required for Nursing but Is Not Essential for Parturition or Reproductive Behaviour,” Proceedings of the National Academy of Sciences of USA, Vol. 93, No. 21, 1996, pp. 11699-11704. doi:10.1073/pnas.93.21.11699
- R. M. J. Deacon, L. L. Cholerton, K. Talbot, R. NairRoberts, D. J. Sanderson and C. Romberg, “Age-Dependent and -Independent Behavioural Deficits in Tg2576 Mice,” Behavioural Brain Research, Vol. 189, No. 1, 2008, pp. 126-138. doi:10.1016/j.bbr.2007.12.024
- R. Deacon, “Assessing Burrowing, Nest Construction, and Hoarding in Mice,” Journal of Visualized Experiments, Vol. 59, 2012, e2607.
- E. Giacalone, M. Tansella, L. Valzelli and S. Garattini, “Brain Serotonin Metabolism in Isolated Aggressive Mice,” Biochemical Pharmacology, Vol. 17, No. 7, 1968, pp. 1315-1327. doi:10.1016/0006-2952(68)90069-5
- J. P. Scott, “Agonistic Behaviour of Mice and Rats: A Review,” American Zoologist, Vol. 6, No. 4, 1966, pp. 683-701.
- J. N. Crawley and R. Paylor, “A Proposed Test Battery and Constellations of Specific Behavioural Paradigms to Investigate the Behavioural Phenotypes of Transgenic and Knockout Mice,” Hormones and Behavior, Vol. 31, No. 3, 1997, pp. 197-211. doi:10.1006/hbeh.1997.1382
- J. N. Crawley, W. M. Schleidt and J. F. Contrera, Does social environment decrease propensity to fight in male mice? Behavioral Biology, Vol. 15, No. 1, 1975, pp. 73- 83. doi:10.1016/S0091-6773(75)92105-7
- K. A. Miczek, E. Weerts, M. Haney and J. Tidey, “Neurobiological Mechanisms Controlling Aggression: Preclinical Developments for Pharmacotherapeutic Interventions,” Neuroscience & Biobehavioral Reviews, Vol. 18, No. 1, 1994, pp. 97-110. doi:10.1016/0149-7634(94)90040-X
- S. E. Jones and P. F. Brain, “Performances of Inbred and Outbred Laboratory Mice in Putative Tests of Aggression,” Behavior Genetics, Vol. 17, No. 1, 1987, pp. 87-96. doi:10.1007/BF01066013
- P. Moretti, J. A. Bouwknecht, R. Teague, R. Paylor and H. Y. Zoghbi, “Abnormalities of Social Interactions and Home-Cage Behaviour in a Mouse Model of Rett Syndrome,” Human Molecular Genetics, Vol. 14, No. 2, 2005, pp. 205-220. doi:10.1093/hmg/ddi016
- R. J. Nelson, G. E. Demas, P. L. Huang, M. C. Fishman, V. L. Dawson and T. M. Dawson, “Behavioural Abnormalities in Male Mice Lacking Neuronal Nitric Oxide Synthase,” Nature, Vol. 378, 1995, pp. 383-386. doi:10.1038/378383a0
- R. Avitsur, S. G. Kinsey, K. Bidor, M. T. Bailey, D. A. Padgett and J. F. Sheridan, “Subordinate Social Status Modulates the Vulnerability to the Immunological Effects of Social Stress,” Psychoneuroendocrinology, Vol. 32, No. 8-10, 2007, pp. 1097-1105. doi:10.1016/j.psyneuen.2007.09.005
- R. Avitsur, J. L. Stark and J. F. Sheridan, “Social Stress Induces Glucocorticoid Resistance in Subordinate Animals,” Hormones and Behavior, Vol. 39, No. 4, 2001, pp. 247-257. doi:10.1006/hbeh.2001.1653
- R. C. La Barba, J. Martini and J. White, “The Effect of Maternal-Separation on the Growth of Ehrlich Carcinoma in the Balb-c Mouse,” Psychosomatic Medicine, Vol. 31, No. 2, 1969, pp. 129-133.
- R. D. Romeo, A. Mueller, H. M. Sisti, S. Ogawa, B. S. McEwen and W. G. Brake, “Anxiety and Fear Behaviours in Adult Male and Female C57BL/6 Mice Are Modulated by Maternal Separation,” Hormones and Behavior, Vol. 43, No. 5, 2003, pp. 561-567. doi:10.1016/S0018-506X(03)00063-1
- J. H. Van Heerden, V. Russell, A. Korff, D. J. Stein and N. Illing, “Evaluating the Behavioural Consequences of Early Maternal Separation in Adult C57BL/6 Mice: The Importance of Time,” Behavioural Brain Research, Vol. 207, No. 2, 2010, pp. 332-342. doi:10.1016/j.bbr.2009.10.015
- J. B. Panksepp and G. P Lahvis, “Social Reward among Juvenile Mice,” Genes, Brain and Behavior, Vol. 6,No. 7, 2007, pp. 661-671. doi:10.1111/j.1601-183X.2006.00295.x
- C. Rondinini, A. Venerosi, I. Branchi, G. Calamandrei and E. Alleva, “Long-Term Effects of Prenatal 3’-Azido- 3’-Deoxythymidine (AZT) Exposure on Intermale Aggressive Behaviour of Mice,” Psychopharmacology, Vol. 45, No. 3, 1999, pp. 317-323. doi:10.1007/s002130051064
- L. J. Keeling and H. W. Gonyou, “Social Behaviour in Farm Animals,” CABI Publishing, London, 2001. doi:10.1079/9780851993973.0000
- M. L. Terranova and G. Laviola, “Delta-Opioid Modulation of Social Interactions in Juvenile Mice Weaned at Different Ages,” Physiology & Behavior, Vol. 73, No. 3, 2001, pp. 393-400. doi:10.1016/S0031-9384(01)00447-4
- T. Miyakawa, M. Yamada, A. Duttaroy and J. Wess, “Hyperactivity and Intact Hippocampus-Dependent Learning in Mice Lacking the M1 Muscarinic Acetylcholine Receptor,” Journal of Neuroscience, Vol. 21, No. 14, 2001, pp. 5239-5250.
- T. Ishihama, Y. Ago, N. Shintani, H. Hashimoto, A. Baba and K. Takuma, “Environmental Factors during Early Developmental Period Influence Psychobehavioural Abnormalities in Adult PACAP-Deficient Mice,” Behavioural Brain Research, Vol. 209, No. 2, 2010, pp. 274-280. doi:10.1016/j.bbr.2010.02.009
- C. M. Spencer, O. Alekseyenko, E. Serysheva, L. YuvaPaylor and R. Paylor, “Altered Anxiety-Related and Social Behaviours in the Fmr1 Knockout Mouse Model of Fragile X Syndrome,” Genes, Brain and Behavior, Vol. 4, No. 7, 2005, pp. 420-430. doi:10.1111/j.1601-183X.2005.00123.x
- M. A. Mines, C. J. Yuskaitis, M. K. King, E. Beurel and R. S. Jope, “GSK3 Influences Social Preference and Anxiety-Related Behaviours during Social Interaction in a Mouse Model of Fragile X Syndrome and Autism,” PLoS One, Vol. 5, No. 3, 2010, e9706. doi:10.1371/journal.pone.0009706
- L. H. Turner, C. E. Lim and S. C. Heinrichs, “Antisocial and Seizure Susceptibility Phenotypes in an Animal Model of Epilepsy Are Normalized by Impairment of Brain Corticotropin-Releasing Factor,” Epilepsy & Behavior, Vol. 10, No. 1, 2007, pp. 8-15. doi:10.1016/j.yebeh.2006.08.013
- A. Siegmund and C. T. Wotjak, “A Mouse Model of Posttraumatic Stress Disorder That Distinguishes between Conditioned and Sensitised Fear,” Journal of Psychiatric Research, Vol. 41, No. 10, 2007, pp. 848-860. doi:10.1016/j.jpsychires.2006.07.017
- C. Zanettini, V. Carola, L. Lo Iacono, A. Moles, C. Gross and F. R. D’Amato, “Postnatal Handling Reverses Social Anxiety in Serotonin Receptor 1A Knockout Mice,” Genes, Brain and Behavior, Vol. 9, No. 1, 2010, pp. 26-32. doi:10.1111/j.1601-183X.2009.00531.x
- H. Tsunekawa, Y. Noda, M. Miyazaki, F. Yoneda, T. Nabeshima and D. Wang, “Effects of (R)-(-)-1-(benzofuran-2-yl)-2-propylaminopentane Hydrochloride [(-)-BPAP] in Animal Models of Mood Disorders,” Behavioural Brain Research, Vol. 189, No. 1, 2008, pp. 107-116. doi:10.1016/j.bbr.2007.12.016
- D. K. Pandey, R. Mahesh, A. A. Kumar, V. S. Rao, M. Arjun and R. Rajkumar, “A Novel 5-HT(2A) Receptor Antagonist Exhibits Antidepressant-Like Effects in a Battery of Rodent Behavioural Assays: Approaching EarlyOnset Antidepressants,” Pharmacology Biochemistry and Behavior, Vol. 94, No. 3, 2010, pp. 363-373. doi:10.1016/j.pbb.2009.09.018
- M. Filali and R. Lalonde, “Age-Related Cognitive Decline and Nesting Behaviour in an APPswe/PS1 Bigenic Model of Alzheimer’s Disease,” Brain Research, Vol. 2009, pp. 1292:93-99. doi:10.1016/j.brainres.2009.07.066
- M. Filali, R. Lalonde and S. Rivest, “Anomalies in Social Behaviors and Exploratory Activities in an APPswe/PS1 Mouse Model of Alzheimer’s Disease,” Physiology & Behavior, Vol. 104, No. 5, 2011, pp. 880-885. doi:10.1016/j.physbeh.2011.05.023
- M. Filali and R. Lalonde, “The Effects of Subchronic D-Serine on Left-Right Discrimination Learning, Social Interaction, and Exploratory Activity in APPswe/PS1 Mice,” European Journal of Pharmacology, 2012, in Press.
- S. Pietropaolo, P. Delage, F. Lebreton, W. E. Crusio and Y. H. Cho, “Early Development of Social Deficits in APP and APP-PS1 Mice,” Neurobiology of Aging, Vol. 33, No. 1002, 2012, pp. e17-e27.
- S. S. Moy, J. J. Nadler, N. B. Young, R. J. Nonneman, S. K. Segall and G. M. Andrade, “Social Approach and Repetitive Behaviour in Eleven Inbred Mouse Strains,” Behavioural Brain Research, Vol. 191, No. 1, 2008, pp. 118-129. doi:10.1016/j.bbr.2008.03.015
- C. L. Yochum, P. Bhattacharya, L. Patti, O. Mirochnitchenko and G. C. Wagner, “Animal Model of Autism Using GSTM1 Knockout Mice and Early Post-Natal Sodium Valproate Treatment,” Behavioural Brain Research, Vol. 210, No. 2, 2010, pp. 202-210. doi:10.1016/j.bbr.2010.02.032
- K. Karelina, G. J. Norman, N. Zhang and A. C. DeVries, “Social Contact Influences Histological and Behavioural Outcomes Following Cerebral Ischemia,” Experimental Neurology, Vol. 220, No. 2, 2009, pp. 276-282. doi:10.1016/j.expneurol.2009.08.022
- [61] J. B. Williams, D. Pang, B. Delgado, M. Kocherginsky, M. Tretiakova and T. Krausz, “A Model of Gene-Environment Interaction Reveals Altered Mammary Gland Gene Expression and Increased Tumor Growth Following Social Isolation,” Cancer Prevention Research, Vol. 2, 2009, pp. 850-861. doi:10.1158/1940-6207.CAPR-08-0238
- [62] V. J. Bolivar, S. R. Walters and J. L. Phoenix, “Assessing Autism-Like Behaviour in Mice: Variations in Social Interactions among Inbred Strains,” Behavioural Brain Research, Vol. 176, No. 1, 2007, pp. 21-26. doi:10.1016/j.bbr.2006.09.007
- [63] J. M. Ho, J. H. Murray, G. E. Demas and J. L. Goodson, “Vasopressin Cell Groups Exhibit Strongly Divergent Responses to Copulation and Male-Male Interactions in Mice,” Hormones and Behavior, Vol. 58, No. 3, 2010, pp. 368-377. doi:10.1016/j.yhbeh.2010.03.021
- [64] C. E. Kovacsics and T. D. Gould, “Shock-induced Aggression in Mice Is Modified by Lithium,” Pharmacology Biochemistry and Behavio, Vol. 94, No. 3, 2010, pp. 380- 386. doi:10.1016/j.pbb.2009.09.020
- [65] V. M. Linck, A. L. da Silva, M. Figueiró, E. B. Caramão, P. R. H. Moreno and E. Elisabetsky, “Effects of Inhaled Linalool in Anxiety, Social Interaction and Aggressive Behaviour in Mice,” Phytomedicine, Vol. 17, 2010, pp. 679-683. doi:10.1016/j.phymed.2009.10.002
- [66] J. L. Gariépy, P. L. Gendreau, R. B. Cairns and M. H. Lewis, “D1 Dopamine Receptors and the Reversal of Isolation-Induced Behaviours in Mice,” Behavioural Brain Research, Vol. 95, No. 1, 1998, pp. 103-111. doi:10.1016/S0166-4328(97)00215-5
- [67] T. Miyakawa, L. M. Leiter, D. J. Gerber, R. R. Gainetdinov, T. D. Sotnikova and H. Zeng, “Conditional Calcineurin Knockout Mice Exhibit Multiple Abnormal Behaviours Related to Schizophrenia,” Proceedings of the National Academy of Sciences of USA, Vol. 100, No. 15, 2003, pp. 8987-8992. doi:10.1073/pnas.1432926100
- [68] Z. Callaerts-Vegh, T. Beckers, S. M. Ball, F. Baeyens, P. F. Callaerts and J. F. Cryan, “Concomitant Deficits in Working Memory and Fear Extinction Are Functionally Dissociated from Reduced Anxiety in Metabotropic Glutamate Receptor 7-Deficient Mice,” Journal of Neuroscience, Vol. 26, No. 24, 2006, pp. 6573-6582. doi:10.1523/JNEUROSCI.1497-06.2006
- [69] T. Maekawa, S. Kim, D. Nakai, C. Makino, T. Takagi and H. Ogura, “Social Isolation Stress Induces ATF-7 Phosphorylation and Impairs Silencing of the 5-HT 5B Receptor Gene,” EMBO Journal, Vol. 29, 2010, pp. 196-208. doi:10.1038/emboj.2009.318
NOTES
*Corresponding author.

