Advances in Microbiology
Vol.4 No.10(2014), Article ID:49239,14 pages
DOI:10.4236/aim.2014.410072
Batch, Fed Batch Production and Characterization of Glutaminase Free L-Asparaginase II of Pectobacterium carotovorum MTCC 1428 in Escherichia coli
Rachna Goswami, Krishnamoorthy Hegde, Venkata Dasu Veeranki*
Biochemical Engineering Laboratory, Department of Biotechnology, Indian Institute of Technology Guwahati, Guwahati, Assam, India
Email: *veeranki@iitg.ernet.in
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 7 June 2014; revised 18 July 2014; accepted 2 August 2014
ABSTRACT
The present study describes the production optimization of recombinant L-asparaginase II of Pectobacterium carotovorum MTCC 1428 in Escherichia coli BL21 (DE3) at batch and fed batch bioreactor level. Production of recombinant L-asparaginase II in batch and fed batch mode was found to be 1.34 and 5.38 folds higher, respectively as compared to shake flask culture. SDS-PAGE and native PAGE of the purified enzyme revealed that molecular mass of the subunits and native enzyme are ~37.5 kDa and ~150 kDa, respectively. Optimum range of pH and temperature for hydrolysis of L-asparagine were found to be 7.5 - 8.5 and 47˚C - 52˚C, respectively. The recombinant enzyme is very specific for its natural substrate, L-asparagine. The activity of recombinant L-asparaginase II is improved by mono cations and diverse effectors including Na+, K+, L-cystine, L-histidine, glutathione and 2-mercaptoethanol whereas, it is moderately inhibited by different divalent cations and thiol group blocking reagent. The kinetic parameters Km, Vmax, kcat and Km/Kcat of purified recombinant L-asparaginase II were determined. The purified L-asparaginase II possesses no partial glutaminase activity, which is prerequisite to reduce the possibility of side effects during the course of anti-cancer therapy.
Keywords:Batch Fermentation, Fed-Batch Fermentation, Recombinant L-Asparaginase II, Taguchi’s Method

1. Introduction
L-asparaginase (EC 3.5.1.1) is used as an antileukimic drug [1] . It hydrolyzes L-asparagine to L-aspartic acid and ammonia. This amino acid deficiency leads to inhibition of RNA and DNA synthesis and causes apoptotic cell death of leukemic cells [1] . L-asparaginase is also used in food industry for the production of acrylamide free starchy food products such as potato chips and biscuits. As a food processing aid, L-asparaginase can efficiently reduce the level of acrylamide up to 90% in an array of starchy foods without changing the flavor and emergence of the end product [2] .
L-asparaginase production has been accounted from many microorganisms [3] , plants [4] and yeast [5] . However, the production of L-asparaginase from wild strain is extremely inadequate. Therefore, recombinants L-asparaginases were developed to enhance the expression of L-asparaginase [6] [7] . The production of any metabolite differs from shake flask to bioreactor system. This might be due to agitation pattern, uncontrolled pH and aeration in shake flask fermentation [8] . The method of studying one variable at a time, while keeping all others at a predetermined level is very inefficient and time consuming technique [8] [9] . Factorial designs are not feasible to optimize parameters in bioreactors because the experiments cannot be performed in blocks. Further, they require more information to design parameter levels [10] . Taguchi’s method has been used extensively in industrial process design, predominantly in developmental trials. This technique is used to engender enough process information to establish the screening and most favorable conditions of parameters for particular procedure with a minimum number of experiments possible. The fundamental principle of this system serves as screening filters which examine the effects of many process variables and recognize those factors, which have major effects on process using a small number of experiments [8] . Most of the L-asparaginases (except the one occurring in guinea pig serum) possess glutaminase activity, which is up to 9% of the L-asparaginase activity and basis of side effects such as serious liver disorders, acute pancreatitis, hyperglycemia and immunosuppression [11] . Glutamine is the key transport form of amino nitrogen in the blood and also an amino group contributor for several biosynthetic reactions. As a result, low plasma glutamine levels causes impairment in a variety of biochemical functions [12] . The interest in P. carotovorum MTCC 1428 L-asparaginase II arose from the information that it does not possess gulataminase activity [13] . Among two types of L-asparaginase: Type I and Type II (found in E. coli B), Type II L-asparaginase has higher affinity towards asparagine than Type I L-asparaginase. So, L-asparaginase II has been studied extensively to use in chemotherapy. This work represents an imperative step in the optimization of fermentation conditions for production of glutaminase free L-asparaginase II of P. carotovorum MTCC 1428 in E. coli BL21 (DE3) in bioreactor. Taguchi’s L9 orthogonal array (OA) was employed to optimize the production conditions in batch bioreactor.
The final quality of biotechnological products is determined at the purification level, which may be regarded as the most important stage in the entire production process. This necessity is even more crucial for remedial products such as therapeutic enzymes, vaccines and antibiotics that require a very high purity level [14] . In addition, characterization of an enzyme is prerequisite to comprehend the properties of enzyme. Therefore, we have purified and characterized the recombinant L-asparaginase II and evaluated the effect of pH, temperature, time of incubation, ionic strength of buffer, different metals ions and inhibitors on enzyme activity. Moreover, substrate specificity of enzyme with different substrate analogues has been studied and kinetic parameters (Km, Kcat and Km/Kcat) were also determined.
2. Materials and Methods
2.1. Chemicals
Isopropyl-β-d-thiogalactopyranoside (IPTG) and ampicillin were procured from Sigma, India. L-asparagine and ammonium sulfate, culture media and their components were purchased from Hi-Media, India. Nessler’s reagent was purchased from Lobacompany, India. All chemicals were purchased from Sigma unless otherwise stated and were of the highest quality available.
2.2. Bacterial Strain and Plasmid
The strain used in this study was ampicillin-resistant recombinant E. coli BL21 (DE3) developed for over-expression of the gene encoding for L-asparaginase II protein of P. carotovorum MTCC 1428. Plasmid used in this study was pET 22 b(+) with Ampicillin resistant marker (Novagen , USA). The amplified L-asparaginase II gene of P. carotovorum MTCC 1428 was cloned between BamHI and XhoI restriction sites of pET 22 b(+) in the downstream of the T7 promoter and resulting recombinant construct was transformed in E. coli BL21 (DE3) for expression of recombinant L-asparaginase II. The over-expression of cloned gene was under the regulation of T7 polymerase responsive promoter. It was maintained in 20% sterile glycerol at −80˚C.
2.3. Inoculum Development
A loop full of frozen glycerol stock (kept at −80˚C) was streaked on a LB-agar plate supplemented with ampicillin (100 µg/ml) and incubated at 37˚C for 14 - 16 h. A single isolated colony was transferred in 20.0 ml of sterile LB medium supplemented with 100 µg∙ml−1 ampicillin in a 100 ml Erlenmeyer flask on a rotary shaker at 37˚C and 200 rpm for 6 - 8 h. This pre-inoculum was transferred at a rate of 2.5% (v/v) to the main inoculum medium (tryptone: 13.30 g/l, yeast extract: 6.38 g/l and NaCl: 7.12 g/l).
2.4. Optimization Methodology for Enhanced Production of Recombinant L-Asparaginase II of P. carotovorum MTCC 1428 in Batch Mode of Cultivation
In order to reduce the number of experiments and to optimize the medium components, shake flask experiments were performed using central composite design (data not shown). It is reported that addition of glucose less than 0.05%, improves the plasmid stability and protein yields [15] [16] . Therefore, Taguchi’s method was applied to find out the parameters, which greatly influence the recombinant L-asparaginase II production in a batch bioreactor. According to Taguchi’s orthogonal array of three variables (glucose, controlled pH and DO), nine experiments were performed to evaluate the influence of various parameters on recombinant L-asparaginase II production of P. carotovorum MTCC 1428. The variables and their levels employed in Taguchi’s robust experimental design are given in the Table1 The concentration of glucose, pH (controlled) and DO varied according to the experimental plan given in the Table2 MINITAB® Release 15.1, PA, USA was used in the present in
Table 1 Variables and their levels employed in the Taguchi’s robust design method for optimal L-asparaginase production in a batch bioreactor.
Table 2. Taguchi’s robust experimental design matrix and corresponding recombinant L-asparaginase II production of P. carotovorum MTCC 1428 in E. coli in a batch bioreactor.
vestigation. In each experimental run, the response was recorded as the recombinant L-asparaginase II production (U/ml) and corresponding signal-to-noise (S/N) ratio was calculated using Equation (1) with an objective for estimation of the effects of various parameters on recombinant L-asparaginase II production, where a large S/N ratio is preferred.
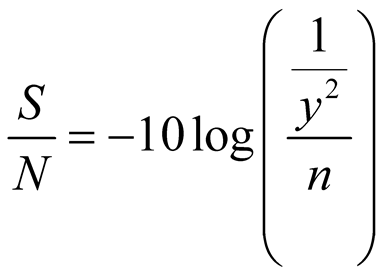 (1)
(1)
where, Y is the response [enzyme activity (U/ml)] and n is the number of experimental runs. Statistical analysis of the results in the form of analysis of variance (ANOVA) was carried out.
2.5. Validation of the Model in a Batch Bioreactor
All batch fermentations were performed in a 3.0 L bioreactor (Applikon, Holland) with 1.0 L working volume of medium. The bioreactor was fitted with necessary controllers. The pH of the medium was adjusted with 2 M NaOH and/or 2 M HCl. The levels of pH (controlled) and The DO was maintained according to experimental plan by varying airflow and impeller speed. Optimum temperature for cell growth was found to be 37˚C whereas for production of recombinant L-asparaginase II it was found to be 30˚C therefore, during growth phase, microorganism was allowed to grow at 37˚C and for production of recombinant L-asparaginase II temperature was reduced to 30˚C. Production of recombinant protein was induced with 1 mM IPTG when cell density at OD 600 nm was 1.50 to 1.80 (~after 3 h of cultivation) and further cultivated for 12 h at 30˚C. The samples were withdrawn at regular intervals of time and enzyme activity was measured in duplicates and averages of the results were taken as response.
2.6. Fed-Batch Cultivation
For production of recombinant L-asparaginase II of P. carotovorum MTCC 1428 in E. coli, fed-batch cultivation was carried out in a 3.0 L bioreactor (Applikon, Holland) starting as a batch culture with a volume of 1.0 L. Fed-batch cultivation was started as batch cultures in previously optimized production conditions. After the exhaustion of glucose, fed-batch experiments were performed by feeding the solutions containing glucose (100 g/l), yeast extract (100 g/l) and ampicillin 100 mg/l. Concentration of glucose in the medium was monitored at regular interval of time and fed by intermittent pulse feeding in proportional to the cell mass [17] -[19] . Feeding of glucose with specific feed rate of 0.5 g glucose per g DCW/h and 0.25 g glucose per g DCW/h was carried out in the growth phase and production phase, respectively and the residual glucose was maintained below 1.0 g/l [19] [20] . The biomass concentration in the bioreactor was calculated throughout the fermentation, by measuring the optical density (OD) as well as the dry cell weight. The DO was controlled between 35% - 40% of saturation by varying agitation (350 - 800 rpm) and aeration (2.0 - 3.0 vvm). The pH was controlled at 7.0 by the addition of 2N HCl and/or 2N NaOH. When the cell OD at 600 nm reached ~10.00, the recombinant protein expression was induced by adding 1mM IPTG at 30˚C and continued for another 12 h.
2.7. Analytical Methods
2.7.1. Assays for L-Asparaginase and L-Glutaminase Activity
L-asparaginase activity was calculated in terms of hydrolysis rate of L-asparagine by determining the quantity of ammonia released in the reaction assay of L-asparaginase. Assay for L-asparaginase and glutaminase was performed as described by Kumar et al. 2009 [13] . The ammonia produced in the reaction was calculated based on a standard curve obtained with ammonium sulfate as standard. One unit of enzyme activity was defined as the amount of enzyme that liberates 1 μmol of ammonia per minute at 37˚C. Specific activity is expressed as units per milligram of protein.
2.7.2. Estimation of Dry Cell Weight (DCW)
The cell density was determined by measuring the culture’s optical density at A600 nm with a UV-visible spectrometer. Dry cell weight was determined by centrifuging the sample broth at 8000 g for 10 min and drying the washed cells to constant weight at 80˚C.
2.7.3. Estimation of Glucose
Residual glucose concentration in the samples was estimated by the 3, 5-dinitrosalicylic acid (DNS) method using glucose as standard [21] .
2.8. Measurement of Plasmid Stability
Samples taken at regular interval of time were diluted appropriately. A diluted sample was spread on a non-selective (amp−) and selective (amp+) LB agar plate. The plate was incubated at 37˚C for 16 h. The ratio of the number of CFU (colony forming units) on the selective agar plate to that on the non-selective agar was used to determine the percentage of plasmid-carrying cells, which was used as an indicator of plasmid stability [15] .
2.9. Enzyme Production and Purification
After 8 h of post induction (fed batch), cells were harvested from 20 ml of culture broth by centrifugation and washed twice with 50 mM Tris-HCl (pH 8.5) and the pellet was suspended in 5.0 ml of 50 mM sodium phosphate buffer (pH 7.0, 500 mM NaCl, 10 mM imidazole), and ultrasonicated on ice at 20 MHz, 30% amplitude, 4 cycles (2 min per cycles with 2 s and 1 s off). After ultrasonication, the contents were centrifuged at 20,000 g for 15 min (4˚C ± 1˚C). The supernatant was loaded onto a 2.0 ml Ni affinity column equilibrated with 50 mM sodium phosphate buffer (pH 8.0, 500 mM NaCl, 10 mM imidazole). After 30 min, the column was washed with 20 ml of the buffer containing 20 mM imidazole. The protein was eluted with 200 mM imidazole and dialyzed in 50 mM Tris-HCl buffer for 24 h. After dialysis, the enzyme activity and protein concentration of the purified L-asparaginase was determined [6] .
2.10. Characterization of Recombinant L-Asparaginase II
2.10.1. Effect of pH on Activity and Stability of Purified Enzyme
The effect of pH on the activity of purified recombinant L-asparaginase II was determined under assay conditions over a pH range of 5.5 - 10.5. For pH stability determination, enzyme preparations were incubated at different pH (5.5 to 10.5) for 24 h at 4˚C ± 1˚C in the absence of substrate and residual activity was determined.
2.10.2. Effect of Incubation Temperature and Time on Activity of Purified Enzyme
To study the effect of incubation temperature on the activity of recombinant L-asparaginase II, enzyme assay was performed at different temperatures ranging from 27˚C to 57˚C. To study the effect of the incubation time, enzyme was incubated separately with substrate for 15 to 90 minutes under the standard conditions and then enzyme activity was measured.
2.10.3. Effect of Ionic Strength
To determine the effect of ionic strength on the activity of recombinant L-asparaginase II, the activity of the enzyme was determined at different levels of ionic strength of buffer (5 to 100 mM).
2.10.4. Effect of Various Effectors on Enzyme Activity
The enzyme activity was determined in the presence of different effectors as shown in Table5 In this study, the concentration of different effectors has been taken on the basis of concentration reported by various researchers for characterization of L-asparaginase [22] . After 30 minutes exposure to the each effecter, the relative activity was expressed as the percentage ratio of the activity of the enzyme incubated with the effectors to that of the untreated enzyme. All of the metal ions (Hg2+, Co2+, Ca2+, Zn2++, K2+, Fe3+, Zn2+, Ni2+, Cd2+, Mn2+, and Mg2+) were in the form of chloride.
2.10.5. Substrate Specificity
The activity of recombinant L-asparaginase II was monitored using various substrates (at a final concentration of 10 mM) viz., D-asparagine, DL-asparagine, L-glutamine, D-glutamine, D-aspartic acid, DL-aspartic acid, Lglutamic acid, succinamic acid, L-aspartic acid amide, L-asparagine-t-butyl ester HCl, BOC-L-asparagine and N-α-acetyl-L-asparagine.
2.10.6. Determination of Kinetic Parameters
The Michaelis Menten constant (Km), maximal velocity (Vmax) and turnover numbers (kcat) of the purified recombinant L-asparaginase II was determined using L-asparagine as substrate in the range of 0.05 to 2.5 mM. The reported reaction velocity is the mean of at least four measurements, which were normalized relative to a blank. The kinetic parameters Km, Vmax and kcat were calculated by non-linear regression analysis of experimental steady-state data to the Michaelis-Menten equation using the computer program GraFit, Erithacus Software. Turnover numbers were calculated on the basis of one active site per 37.5 kDa subunit by SDS-PAGE.
3. Results and Discussion
According to Taguchi’s orthogonal array of L9, 9, experiments were performed and results are presented in the Table2 Depending upon the combination of the chemical and physical process variables and their levels, recombinant L-asparaginase II production varied in each run significantly, representing the strong influence of the variables and their levels on the response. To understand the effect of these variables on recombinant L-asparaginase II production, their ranking was performed based on the calculated delta S/N ratio. Generally, delta value for a factor, calculated by measuring the variation between the highest and lowest characteristic average S/N ratio of the factor, specify its comparative significance over others on a given response. Higher value of delta for a factor signifies a larger significant consequence than others, while S/N ratio indicates effect of factors on a response. Thus delta S/N ratio have used as a key factor for ranking the variables for their effects on the response [23] . In this study, based on the delta S/N ratio acquired for each factor, the three parameters were ranked accordingly, which revealed that pH had the maximum effect and followed by glucose concentration and DO (Table 3). To validate these findings on the significance of the individual parameters and their contribution on recombinant L-asparaginase II production, ANOVA of the results was employed. Table 4 present the ANOVA of L-asparaginase II production of P. carotovorum MTCC 1428 in E. coli that illustrates a term for error, the value of mean sum of squares (MS). In general, low P value of a term in ANOVA indicates that high significance of the term. In this study, all the parameters viz., glucose concentration, pH and DO were found to have a considerable effect on recombinant L-asparaginase II production. The effect of parameters on recombinant L-asparaginase II production by ANOVA is in good agreement with those observed earlier from the factors ranking based on their delta S/N ratio.
Table 3. Values of average S/N ratio and their ranking based on delta S/N ratio.
Table 4. Analysis of variance for recombinant L-asparaginase II production of P. carotovorum MTCC 1428.
DF, Degrees of freedom; Seq SS, Sequential sum of squares; Ads SS, Adjusted sum of squares; Ads MS, Adjusted Mean square; R-Sq = 99.9%; R-Sq(adj) = 99.6%.
3.1. Selection of Optimum Levels of Parameters for Enhanced Recombinant L-Asparaginase II Production
The three parameters, glucose, pH (controlled) and DO were analyzed at different levels; particular levels of the variables caused a significant augment in the mean response as compared to other levels of the variables. The level 3 of glucose and DO and level 2 of pH were found to display a remarkable positive consequence on the response. These levels of the variables were therefore preferred as optimum for the maximum production of recombinant L-asparaginase II in the medium. Production of recombinant L-asparaginase II was enhanced in the optimized conditions. At the optimum levels of parameters, recombinant L-asparaginase II production was performed in a batch bioreactor (3 L bioreactor, Applikon, Holland) with 1.0 L medium. It was observed that the production of recombinant L-asparaginase II was very high (23.88 U/ml) in the bioreactor as compared to any of those values shown in Table2 The production of recombinant L-asparaginase was found to be 1.34 fold higher under optimal levels of parameters as compared to shake flask (17.83 U/ml). Due to higher growth of cells in bioreactor (1.81 g/l) than shake flask (1.39 g/l), specific activity of enzyme was lower (63.19 U/mg) than shake flask (67.95 U/mg). However, the overall productivity increased in bioreactor (3411.43 U/l/h) than shake flask (2971.66 U/l/h). Maximum production of recombinant L-asparaginase II was achieved at 7 h of fermentation in a batch bioreactor and plasmid stability analysis was carried out by comparing the number of CFUs (colony forming units) on amp+ and amp− plates formed after plating culture, withdrawn at different time-points during cultivation. The presence of ampicillin resistance was used as an indicator for the presence of recombinant plasmid in the cells. It was found that 79% of the culture retained the recombinant plasmid at 24 h post-induction, similar to the shake flask cultivation.
Due to un-availability of literature on optimization of recombinant L-asparaginase II production by Taguchi’s method, we could not compare the outcome of recombinant L-asparaginase II production of P. carotovorum MTCC 1428 from E. coli. However, there are many reports existing for optimization of production conditions for recombinant and non-recombinant proteins by using Taguchi’s method. Dasu et al. (2003) [8] has optimized the griseofulvin production from Penicillium griseofulvum MTCC 1898 in a batch bioreactor. Niccolai et al. (2003) [24] has optimize the production of recombinant Helicobacter pylori neutrophila activating (NAP) protein from E.coli in bioreactor using Taguchi’s method and an overall 2.91-fold higher production of recombinant NAP was achieved. Hao et al. (2006) [25] have also exploited Taguchi’s method to screen the important medium constituents for optimization of the medium for higher production of cytochrome P450 2C9 from E. coli DH5α. Ghane and Khodabandeh (2008) [26] have produced the 28% higher production of interferon beta from E. coli using Taguchi’s methodology.
3.2. Overproduction of Recombinant L-Asparaginase II in Fed-Batch Culture
Fed-batch culture was carried out to achieve higher production and productivity of recombinant L-asparaginase II in a 3 L bioreactor. In fed-batch, maximum biomass and recombinant L-asparaginase II production were found to be 7.32 g/l dry cell weight and 95.85 U/ml, respectively. It was about four fold higher as compared to batch bioreactor. The activity increased to a maximum value and then began to reduce within 8 h of post-induction. It might be due to significant plasmid loss started after 8 h of post induction (Figure 1). There is no report available for maximization of production of recombinant L-asparaginase II of P. carotovorum MTCC 1428 from E. coli by batch and fed batch culture. Only Khushoo et al. (2005) [27] have produced recombinant L-asparaginase of E. coli in fed batch culture and maximum volumetric yield of 8.7 × 105 units/l was achieved. Giridhar and Srivastava (2000) [18] have achieved higher production of L-sorbose from Acetobacter suboxydans using pulse feeding strategy. Furthermore, higher production of Phytolacca insularis protein in fed-batch culture of recombinant E. coli was achieved by stepwise increasing feeding, according to the biomass [28] . By using stepwise increasing feeding of substrate Zhang et al. (2009) [20] and Gummadi and Bhavya (2011) [29] have reported enhanced production of anticancer drug TATm-survivin (T34A) and caffeine demethylase in Escherichia coli.
3.3. Purification of Recombinant L-Asparaginase II
Purification of recombinant L-asparaginase II was achieved apparent homogeneity using a single step Ni-NTA affinity chromatography with 5.50 folds (288.45 U/mg) and 74% of yield. SDS-PAGE and native PAGE of the purified recombinant asparaginase II inferred that that molecular mass of the subunits and native enzyme is ~37.5 kDa and ~150 kDa, respectively (Figure 2(A) and Figure 2(B)). These results corroborate with the available reports, which revealed that the functional form of bacterial L-asparaginase exists as a tetramer of identical subunits, with molecular mass in the range of 140 - 160 kDa [30] [31] . L-Asparaginase tetramer have considered as a dimmer of dimmers [6] .
3.4. Characterization of Recombinant L-Asparaginase II
3.4.1. Effect of pH on Activity and Stability of Purified Enzyme
Recombinant L-asparaginase II of P. carotovorum MTCC 1428 have shown activity in a broad range of pH of 6.5 - 10.5 with an optimum pH of 8.5. The maximum activity in alkaline pH is most likely due to the equilibrium between L-aspartic acid and L-aspartate. L-aspartic acid in acidic pH has a greater affinity for the active site of the enzyme. Under such conditions, it becomes a competitive inhibitor. In alkaline pH, the equilibrium is shifted toward the aspartate, which is the form with lower affinity to the active site enabling, a favorable balance
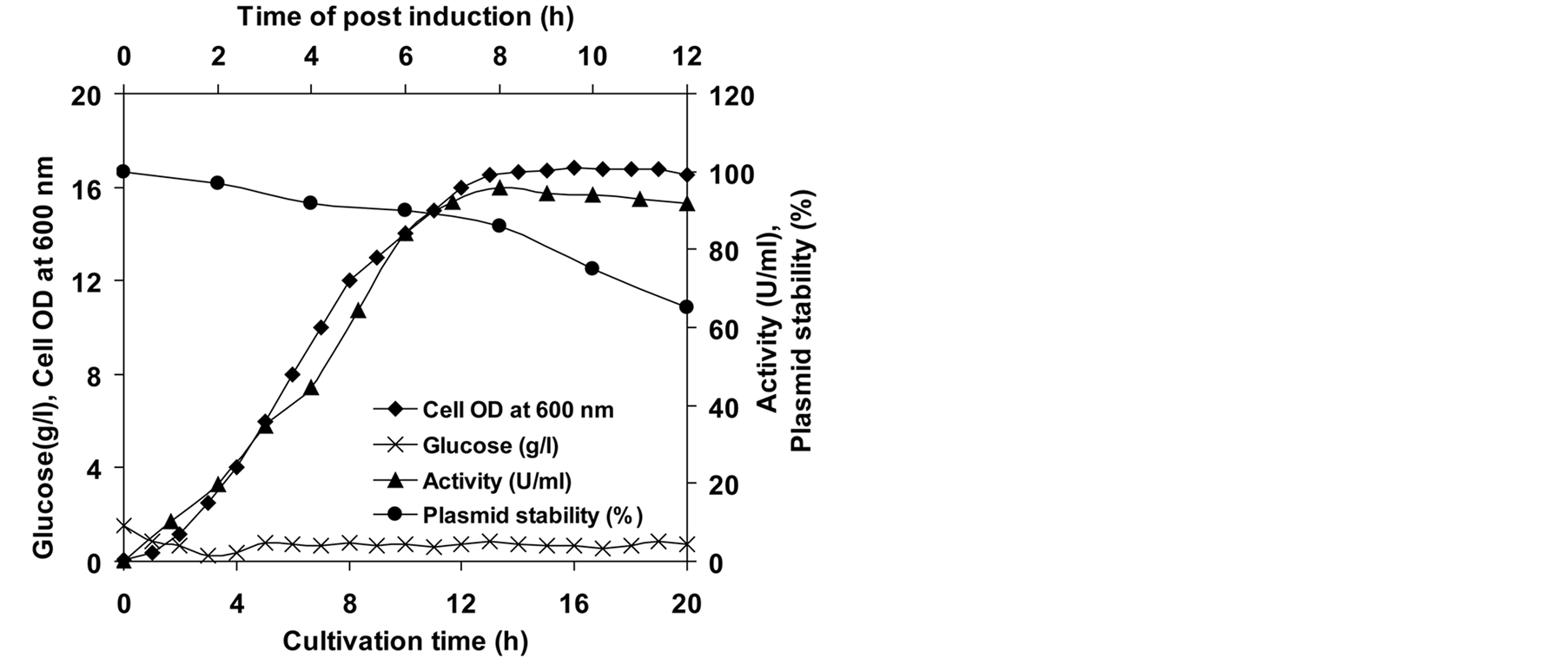
Figure 1. Production of recombinant L-asparaginase II of P. carotovorum MTCC 1428 in E. coli in fed batch culture.


Figure 2. SDS and native PAGE of purified L-asparaginase II of P. carotovorum MTCC 1428 (A) SDS-PAGE (12.5%) of recombinant L-asparaginase II of P. carotovorum MTCC 1428 (Lane 1: pET 22b (+) in E. coli BL21 (DE3) without any insert, Lane 2: Crude extract, Lane 3: purified recombinant L-asparaginase II and Lane 4: Marker. (B) Native PAGE (7.5%) of recombinant L-asparaginase (Lane 1: Purified recombinant L-asparaginase II of P. carotovorum MTCC 1428 and Lane 2: Marker).
for the association with the substrate, L-asparagine [32] . At pH below 6.5 and above 9.5, the enzyme loses approximately 65% and 45% activity, respectively (Figure 3). Though maximum activity at a physiological pH is one of the requisites of L-asparaginase for antitumor activity, by virtue of its broad pH activity profile, about ~85% of the enzyme activity was retained at pH 7.5. The enzyme showed stability at alkaline pH range (pH 7.5 - 9.5) as it retained ~80% of its original activity after incubation for 24 h (Figure 3). Most of the L-asparaginases from Erwinia species showed alkaline pH optima (8.0 - 9.0) except L-asparaginase from E. coli, which displayed acidic pH optimum of 5.0 - 6.0 [11] .
3.4.2. Effect of Incubation Temperature and Time on the Activity
The purified enzyme exhibited maximum activity at a temperature range of 47˚C - 52˚C at pH 8.5. The activity decreased sharply above the optimal temperature range and approximately 75% of its original activity was lost at 57˚C (Figure 4). Probably, enzyme losses activity at temperature above than 50˚C. Maladkar et al. (1993) [33] have observed the optimum temperature of 50˚C for Erwinia L-asparaginase. At lower temperature, the activity is low due to slow reaction rate. We have studied the effect of incubation time on L-asparaginase performance. Highest enzyme activity was observed after 30 minute of incubation time (Figure 5). Incubation time has shown inverse effect on enzyme activity as activity was reduced by increasing the incubation time. El-Sayed et al. (2011) [34] have also observed the decrease in L-asparaginase activity after 90 min. of incubation with substrate. The decrease in activity of enzyme was observed when incubated for longer period of time is due to the product inhibition [35] .
3.4.3. Effect of Ionic Strength on the Activity
As the enzyme activity is altered by ionic strength, it was of interest to consider the effect of tris ions on the affinity of enzyme and substrate by electrostatic interactions of the added ions. The interaction of inorganic ions
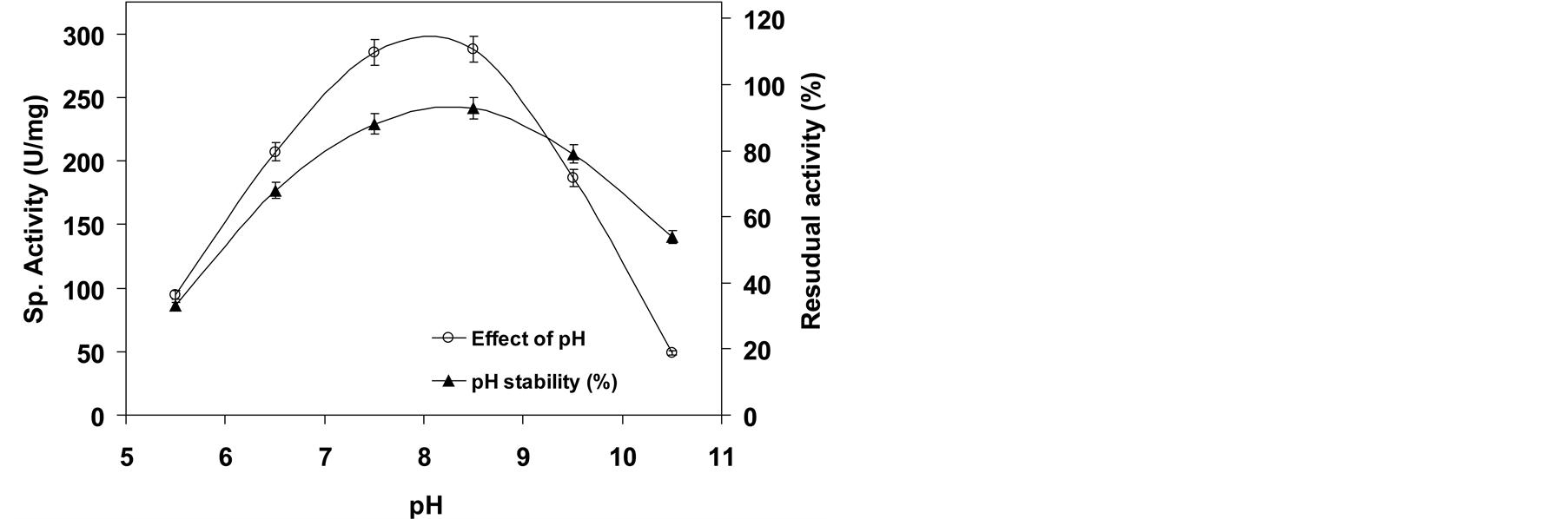
Figure 3. Effect of pH on enzyme activity and stability.
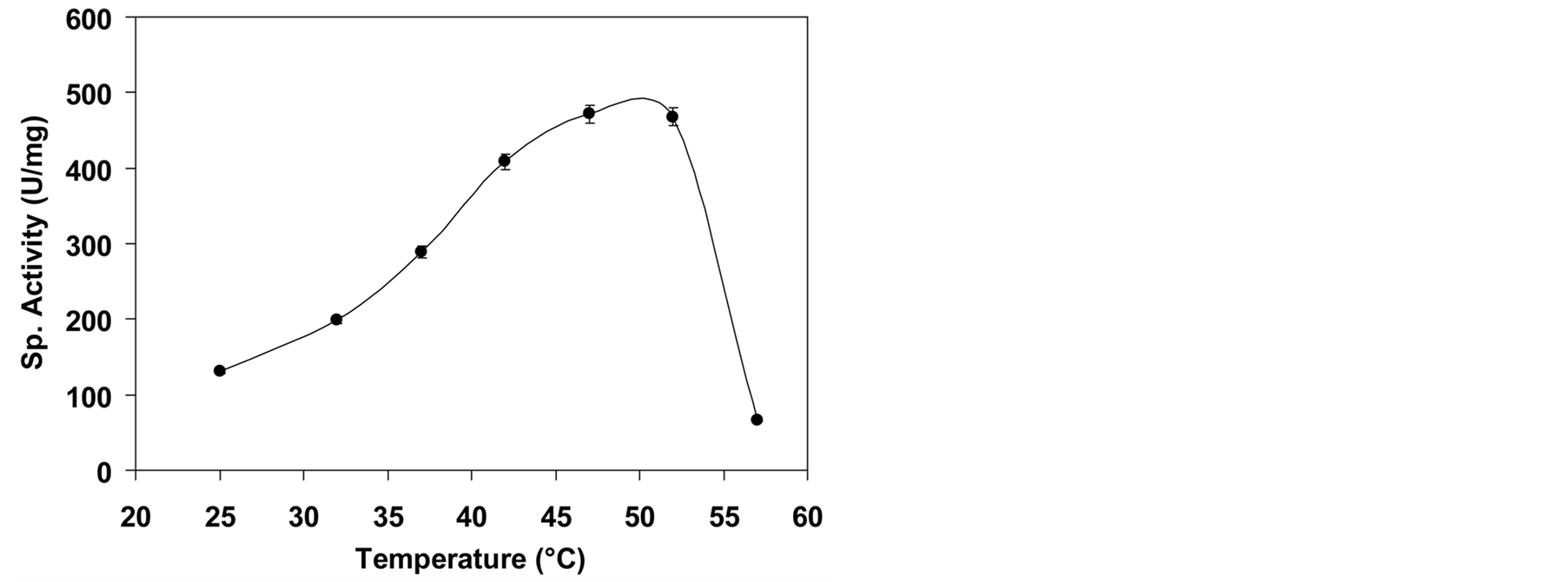
Figure 4. Effect of temperature on enzyme activity.
with the enzyme did not affect the three dimensional shape of the enzyme in a substantial manner however could formulate it easier for a substrate molecule to locate or bind to the active site of the enzyme [36] . We observed that decrease in enzyme activity with both higher as well as lower strength of buffers. Minimum and maximum enzyme hydrolysis occurred at buffer concentration of 5 mM and 50 mM, respectively (Figure 6). Reduction in the performance of enzyme at lower/higher ionic strength of buffer might be due to inability of enzyme to form non covalent interaction with the substrate [36] .
3.4.4. Effect of Various Effectors and Substrate Specificity
Activity of recombinant L-asparaginase II was assayed in the presence of several reagents (Table 5). Amongst the salts tested, significant loss of activity was observed with Hg2+, Ni2+, Cd2+, Co2+ Cu2+, Fe3+, Mg2+ and Zn2+, whereas Na+ and K+ acted as an enhancer, inhibition of enzyme activity with different metal ions and no effect in the presence of the metal chelator, EDTA inferred that it is not a metalloprotein. Inhibition of enzyme activity in presence of Hg2+, Cd2+, and Zn2+ might be due to the of essential vicinal sulfhydryl group(s) of the enzyme for catalysis. Furthermore, the role of sulfhydryl groups in the catalytic activity of the enzyme is also confirmed by the stimulation of activity by the reducing reagents likes 2-mercaptoethanol and glutathione, and inhibition by thiol group blocking reagent, iodoactamide. L-cysteine and histidine found to be stimulator for enzyme activity. The enzyme loses 42% and 78% activity with 2.5 M urea and 2.5 M sodium dodecyl sulfate (SDS), respectively. Thiol reactivity was also reported with the purified L-asparaginase from E. carotovora and P. carotovorum MTCC 1428 [22] . Asparaginase possesses the thiol group binding domain with high affinity towards free-SH group containing effectors and these effectors alter the asparaginase from one conformation to another catalytically more active conformation. The asparaginase activation induced by GSH and Cys supports the hypothe-

Figure 5. Effect of incubation time on enzyme activity.
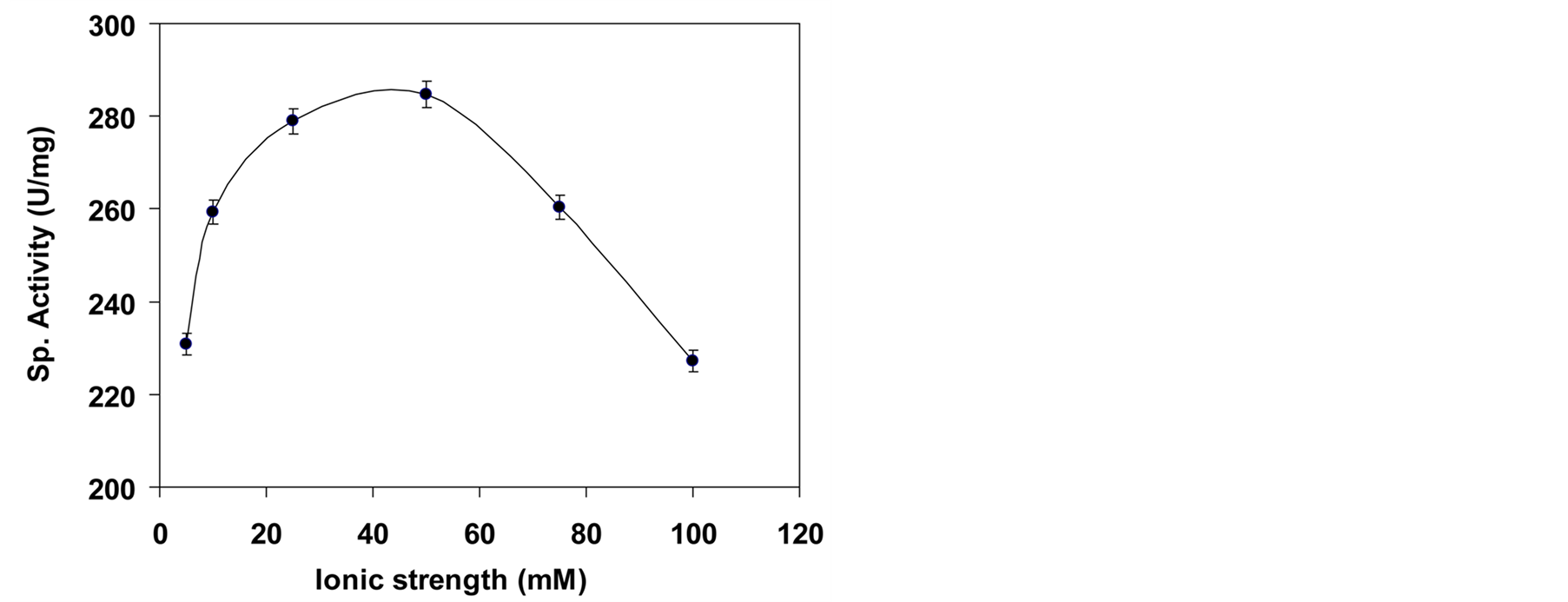
Figure 6. Effect of ionic strength of buffer on enzyme activity.
Table 5. Influence of different effectors on recombinant L-asparaginase II activity.
sis that all thiol group containing compounds and amino acids may combine with the same activator site on asparaginase. Moreover, this kind of stimulatory action has been studied with the glyoxalase and mitogen activated protein kinase [37] .
The substrate specificity of the recombinant enzyme is given in Table5 Only L-asparaginase and no glutaminase activity were observed. The absence of glutaminase activity would minimize the risk factor for successful clinical studies [38] . No positive hydrolysis was observed when L-glutamine, L-aspartic acid, D-aspartic acid, DL-aspartic acid and L-glutamic acid were used separately as substrates. Therefore, the novel purified glutaminase-free recombinant L-asparaginase II reported in this study will be an advantageous and value-added product.
3.5. Kinetic Parameters
The Km and Vmax of purified L-asparaginase II of P. carotovorum MTCC 1428 were found to be 0.666 mM and 303.03 IU/mg, respectively. This indicates high affinity of the enzyme towards substrate. The substrate affinity in terms of Km is very low, which is 5 - 9 times lower than the reported cytosolic bacterial L-asparaginases [22] . Turnover numbers (kcat) and specificity constants (kcat/Km) for recombinant L-asparagine II of P. carotovorum MTCC 1428 were determined to be 1.34 × 102/s and 2.01 × 105/M/s, respectively. L-asparaginase of different microorganisms has different substrate affinities and plays different physiological roles in the enzyme activity. The Km value of L-asparaginase of Pseudornonas stutzeri MB-405A was reported to be 0.145 mM (Manna et al. 1995). Warangkar and Khobragade (2010) [39] have reported L-asparaginase from E. carotovora, with lower Km value of 0.09 mM. The kinetic parameters determined in this study were comparable with those reported for many bacterial recombinant L-asparaginases [1] [7] .
4. Conclusion
Production of recombinant L-asparaginase II of P. carotovorum MTCC 1428 in E. coli was carried out in batch and fed batch in bioreactor. Recombinant L-asparaginase II was purified and effect of different effectors on the activity and substrate specificity of recombinant L-asparaginase II was studied. Furthermore, purified recombinant L-asparaginase II has shown no glutaminase activity indicating it to be a potential candidate for therapeutic purpose.
Acknowledgements
The authors gratefully acknowledge Department of Biotechnology, Government of India for financial support.
References
- Kotzia, G.A. and Labrou, N.E. (2005) Cloning, Expression and Characterization of
Erwinia carotovora L-Asparaginase. Journal of Biotechnology, 119, 309-323.
http://dx.doi.org/10.1016/j.jbiotec.2005.04.016 -
Hendriksen, H.V., Kornbrust, B.A., Ostergaard, P.R. and Mary, A. (2009) Evaluating
the Potential for Enzymatic Acrylamide Mitigation in a Range of Food Products Using
an Asparaginase from Aspergillus oryzae. Journal of Agricultural and Food Chemistry,
57, 4168-4176.
http://dx.doi.org/10.1021/jf900174q - Peterson, R.E. and Ciegler, A. (1969) L-Asparaginase Production by Erwinia aroideae. Applied Microbiology, 18, 64-67.
- Verma, N., Kumar, K., Kaur, G. and Anand, S. (2007) L-Asparaginase, A Promising Chemotherapeutic Agent. Critical Reviews in Biotechnology, 27, 45-62. http://dx.doi.org/10.1080/07388550601173926
- Dunlop, P.C., Roon, R.J. and Even, H.L. (1976) Utilization of D-L-Asparaginase by S. cerevisiae. Journal of Bacteriology, 125, 999-1004.
- Khushoo, A., Pal, Y., Singh, B.N. and Mukherjee, K.J. (2004) Extracellular Expression and Single Step Purification of Recombinant Escherichia coli L-Asparaginase II. Protein Expression and Purification, 38, 29-36. http://dx.doi.org/10.1016/j.pep.2004.07.009
-
Kotzia, G.A. and Labrou, N.E. (2007) L-Asparaginase from Erwinia chrysanthemi 3937,
Cloning, Expression and Characterization. Journal of Biotechnology, 127, 657-669.
http://dx.doi.org/10.1016/j.jbiotec.2006.07.037 - Dasu, V.V., Panda, T. and Chidambaram, M. (2003) Determination of Significant Parameters for Improved Griseofulvin Production in a Batch Bioreactor by Taguchi’s Method. Process Biochemistry, 38, 877-880. http://dx.doi.org/10.1016/S0032-9592(02)00068-7
- Stowe, R.A. and Mayer, R.P. (1999) Efficient Screening of Process Variables. Industrial & Engineering Chemistry Research, 56, 36-40.
- Panda, T., Babu, P.S.R., Kumari, J.A., Rao, D.S., Theodore, K. and Jagannandha, K., et al. (1997) Bioprocess Optimization Challenge. Journal of Microbiology and Biotechnology, 7, 367-72.
- Muller, H.J. and Boos, J. (1998) Use of L-Asparaginase in Childhood ALL. Critical Reviews in Oncology/Hematology, 28, 97-113. http://dx.doi.org/10.1016/S1040-8428(98)00015-8
- Lappa, K., Kotzia, G.A. and Labrou, N.E. (2010) Labrou Studies on the Thermal Stability of the Therapeutic Enzyme L-Asparaginase from Erwinia carotovora. Biochemistry Research Trends Series, Nova Science Publisher, New York, 323-334.
- Kumar, S., Pakshirajan, K. and Dasu, V.V. (2009) Development of Medium for Enhanced Production of Glutaminase-Free L-Asparaginase from Pectobacterium carotovorum MTCC 1428. Applied Microbiology and Biotechnology, 84, 477-486. http://dx.doi.org/10.1007/s00253-009-1973-0
- Squez-Alvarez, E., Lienqueo, M.E. and Pinto, J.M. (2001) Optimal Synthesis of Protein Purification Processes. Biotechnology Progress, 17, 685-696. http://dx.doi.org/10.1021/bp010031d
- Zhang, Y.K., Li, T.M. and Liu, J.J. (2003) Low Temperature and Glucose Enhanced T7 RNA Polymerase-Based Plasmid Stability for Increasing Expression of Glucagon-Like Peptide-2 in Escherichia coli. Protein Expression and Purification, 29, 132-139. http://dx.doi.org/10.1016/S1046-5928(03)00002-0
- Studier, F.W. (2005) Protein Production by Auto-Induction in High-Density Shaking Cultures. Protein Expression and Purification, 41, 207-234. http://dx.doi.org/10.1016/j.pep.2005.01.016
- Nayak, D.P. and Vyas, V.V. (1999) Improved Stability and Expression of a Recombinant Shuttle Plasmid in Escherichia coli during Fedbatch Cultivation. World Journal of Microbiology and Biotechnology, 15, 65-71. http://dx.doi.org/10.1023/A:1008870528336
- Giridhar, R. and Srivastava, A.K. (2000) Fed-Batch Sorbose Fermentation Using Pulse and Multiple Feeding Strategies for Productivity Improvement. Biotechnology and Bioprocess Engineering, 5, 340-344. http://dx.doi.org/10.1007/BF02942209
- Ramalingama, S., Gautama, P., Mukherjee, K.J. and Jayaraman, G. (2007) Effects of Post-Induction Feed Strategies on Secretory Production of Recombinant Streptokinase in Escherichia coli. Biochemical Engineering Journal, 33, 34-41. http://dx.doi.org/10.1016/j.bej.2006.09.019
- Zhang, H., Zheng, Y., Liu, Q., Tao, X., Zheng, W., Ma, X. and Wei, D. (2009) Development of a Fed-Batch Process for the Production of Anticancer Drug TATm-Survivin(T34A) in Escherichia coli. Biochemical Engineering Journal, 43, 163-168. http://dx.doi.org/10.1016/j.bej.2008.09.013
- Miller, G.L. (1959) Use of DNS Reagent for Determination of Reducing Sugar. Analytical Chemistry, 31, 426-428. http://dx.doi.org/10.1021/ac60147a030
- Kumar, S., Dasu, V. and Pakshirajan, K. (2011) Purification and Characterization of Glutaminase-Free L-Asparaginase from Pectobacterium carotovorum MTCC 1428. Bioresource Technology, 102, 2077-2082. http://dx.doi.org/10.1016/j.biortech.2010.07.114
- Daverey, A. and Pakshirajan, K.K. (2010) Kinetics of Growth and Enhanced Sophorolipids Production by Candida bombicola Using a Low-Cost Fermentative Medium. Applied Biochemistry and Biotechnology, 160, 2090-2101. http://dx.doi.org/10.1007/s12010-009-8797-3
- Niccolai, A., Fontani, S., Kapat, A. and Olivieri, R. (2003) Maximization of Recombinant Helicobacter pylori Neutrophil Activating Protein Production in Escherichia coli: Improvement of a Chemically Defined Medium Using Response Surface Methodology. FEMS Microbiology Letters, 221, 257-262. http://dx.doi.org/10.1016/S0378-1097(03)00184-8
- Hao, D.C., Zhu, P.H., Yang, S.L. and Yang, L. (2006) Optimization of Recombinant Cytochrome P450 2C9 Protein Production in Escherichia coli DH5α by Statistically-Based Experimental Design. World Journal of Microbiology and Biotechnology, 22, 1169-1176. http://dx.doi.org/10.1007/s11274-006-9158-9
- Ghane, M., Yakhchali, B. and Khodabandeh, M. (2008) Over Expression of Biologically Active Interferon Beta Using Synthetic Gene in E. coli. Journal of Sciences, 19, 203-209.
- Khushoo, A., Pal, Y. and Mukherjee, K.J. (2005) Optimization of Extracellular Production of Recombinant Asparaginase in Escherichia coli in Shake-Flask and Bioreactor. Applied Microbiology and Biotechnology, 68, 189-197. http://dx.doi.org/10.1007/s00253-004-1867-0
- Kweon, D.H., Han, N.S., Park, K.M. and Seo, J.H. (2001) Overproduction of Phytolacca insularis Protein in Batch and Fed-Batch Culture of Recombinant Escherichia coli. Process Biochemistry, 36, 537-542. http://dx.doi.org/10.1016/S0032-9592(00)00237-5
- Gummadi, S.N. and Bhavya, B. (2011) Enhanced Degradation of Caffeine and Caffeine Demethylase Production by Pseudomonas sp. in Bioreactors under Fed-Batch Mode. Applied Microbiology and Biotechnology, 91, 1007-1017. http://dx.doi.org/10.1007/s00253-011-3319-y
- Kozak, M., Jasloski, M. and Rohm, K.H. (2000) Preliminary Crystallographic Studies of 425F Mutant of Periplasmic Escherichia coli L-Asparaginase. Acta Biochimica Polonica, 47, 807-814.
- Aghaiypour,
K., Wlodawer, A. and Lubkowski, J. (2001) Structural Basis for the Activity and
Substrate Specificity of Erwinia chrysanthemi L-Asparaginase. Biochemistry, 40,
5655-5664.
http://dx.doi.org/10.1021/bi0029595 - Lubkowski, J., Wlodawer, A., Ammon, H.L., Copeland, T.D. and Swain, A.L. (1994) Structural Characterization of Pseudomonas 7A Glutaminase-Asparaginase. Biochemistry, 33, 10257-10265. http://dx.doi.org/10.1021/bi00200a005
- Maladkar, N.K., Singh, V.K. and Naik, S.R. (1993) Fermentative Production and Isolation of L-Asparaginase from Erwinia carotovora. Hindustan Antibiotics Bulletin, 35, 77-86.
- El-Sayed, M., El-Sayed, S.T., Shousha, W.G., Shehata, A.N. and Hanafy, S.S. (2011) Purification, Characterization and Antitumor Activity of L-Asparaginase from Chicken Liver. Journal of American Science, 1, 439-449.
- Cunningham, T.J., Yao, L.H. and Lucena, A. (2008) Product Inhibition of Secreted Phospholipase A2 May Explain Lysophosphatidylcholines’ Unexpected Therapeutic Properties. Journal of Inflammation, 5, 17. http://dx.doi.org/10.1186/1476-9255-5-17
- Jimnez, E.S.D., Evangelina, L., Torres, J. and Soberon, G. (1964) On the Mechanism of the Effect of Ionic Strength on Crystalline Aldolase Activity. Journal of Biological Chemistry, 239, 4154-4158.
- Ernst, V., Levin, D.H. and London, I.M. (1979) Inhibition of Protein Synthesis Initiation by Oxidized Glutathione: Activation of a Protein Kinase that Phosphorylates the α Subunit of Eukaryotic Initiation Factor 2. Proceedings of the National Academy of Sciences of the United States of America, 75, 4110-4114.
- Manna, S., Sinha, A., Sadhukhan, R. and Chakrabarty, S.L. (1995) Purification, Characterization and Antitumor Activity of L-Asparaginase Isolated from Pseudomonas stutzeri MB-405. Current Microbiology, 30, 291-298. http://dx.doi.org/10.1007/BF00295504
- Warangkar, S.C. and Khobragade, C.N. (2010) Purification, Characterization, and Effect of Thiol Compounds on Activity of the Erwinia carotovora L-Asparaginase. Enzyme Research, 2010, Article ID: 165878. http://dx.doi.org/10.4061/2010/165878
NOTES

*Corresponding author.


