Journal of Biomedical Science and Engineering
Vol.6 No.8A(2013), Article ID:36110,8 pages DOI:10.4236/jbise.2013.68A1007
Hierarchy of mesenchymal stem cells: Comparison of multipotentmesenchymal stromal cells with fibroblast colony forming units
![]()
1Laboratory for Physiology of Hematopoiesis, FGBI National Hematological Scientific Center, Moscow, Russia
2Bone Marrow Transplantation Department, FGBI National Hematological Scientific Center, Moscow, Russia
Email: iranifontova@yandex.ru, loel@mail.ru, bigchel@pochta.ru, nsats@yandex.ru, ndrize@yandex.ru, kuzlara@rambler.ru, elenap@blood.ru, svg@blood.ru
Copyright © 2013 Irina N. Shipounova et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 11 June 2013; revised 16 July 2013; accepted 28 July 2013
Keywords: Mesenchymal Multipotent Stromal Cells (MMSC); Fibroblast Colony Forming Units (CFU-F); Real-Time Polymerase Chain Reaction; Gene Expression Level; Aging
ABSTRACT
The organization of the compartment of mesenchymal stem cells is still obscure. Two types of human stromal precursor cells are known. Both of them are analyzed in in vitro system: mesenchymal multipotent stromal cells (MMSC) and fibroblast colony forming units (CFU-F). The aim of this study was to compare the main characteristics of MMSC and CFU-F derived from the bone marrow of 24 healthy donors. Growth and differentiation parameters, as well as relative expression levels of different genes were analyzed in MMSC and CFU-F. MMSC were cultivated for 5 passages. CFU-F concentration was determined for each bone marrow sample. The data obtained demonstrated the heterogeneity and hierarchical organization of both studied populations of stromal precursor cellsMMSC and CFU-F. These two types of stromal precursor cells turned to be different in most parameters studied. Altogether MMSC seemed to be more immature cells than CFU-F and took up the higher position in hierarchical tree of mesenchymal stem cells. The rate of differentiation and proliferative potential decreased with the donor’s age in both populations MMSC and CFU-F.
1. INTRODUCTION
Mesenchymal stem cells were identified by Arnold Caplanwho gave this name to the plastic-adherent stromal bone marrow cells and described their ability to differentiate into osteoblasts, adipocytes and chondroblasts [1]. By that time, the existence of two distinct types of stem cells in bone marrow hematopoietic and stromal was known. The first direct evidence was that nonhematopoietic, mesenchymal precursor cells were present in the bone marrow originated from the work of Friedenstein and colleagues [2]. These pioneering experiments involved the incubation of bone marrow mononuclear cells in vitro. The presence of highly heterogeneous adherent fraction was seen within a few days. After 3 - 7 days, individual foci of two to four fibroblasts were observed among others. These fibroblasts could differentiate into cells capable to form small deposits of bone or cartilage in vivo. These cells were termed fibroblast colony forming units or CFU-F. Several studies showed that CFU-F were multipotent and could differentiate into osteoblasts, chondroblasts, adipocytes, and even myoblasts (reviewed in [3]). The frequency of CFU-F in bone marrow suspensions varied among species, and the results were influenced by the culture conditions (reviewed in [4]).
The identification of human mesenchymal stem (stromal) cells still depends on in vitro culture systems. Three in vitro systems are generally applied to examine these cells: the CFU-F assay, MMSC, and the cultivation of mesenchymal cell lines [5].
As mesenchymal stem cells’ self-renewing ability was not definitively proven in vivo, the world-wide community agreed to designate them mesenchymal multipotent stromal cells (MMSC) [6,7]. MMSC population is highly heterogeneous and has been shown in vitro to have the hierarchical structure [8-11]. The relationship between MMSC and CFU-F is still obscure. Some authors consider MMSC and CFU-F to be identical [1,12]. Sometimes the MMSC content of bone marrow samples is estimated by the classic (CFU-F) assay [13]. MMSC number in the bone marrow is correlated positively with CFU-F concentration [14].
The aim of this investigation was to study the relationship between MMSC and CFU-F in the context of hierarchy in the compartment of mesenchymal stem cells. Both populations of stromal precursor cells studied in vitro demonstrated high heterogeneity. Taking into consideration the gene expression data, we suggest that the whole MMSC population is represented by more imamture cells than CFU-F and can be positioned higher than CFU-F in mesenchymal stem cells hierarchy.
2. MATERIALS AND METHODS
2.1. Culture and Characterization of MMSC
MMSC were isolated from the bone marrow of 24 donors (12 males and 12 females) ranging in age from 16 to 56 (median: 32 years). CFU-F was analyzed in the same bone marrow samples. Samples were collected after informed consent during aspiration of hematopoietic stem cells for allogeneic transplantation in the Department of Bone Marrow Transplantation. The protocol was approved by the local Medical Ethics Committee.
MMSC were derived from 5 - 10 ml of donor’s bone marrow. For mononuclear cells, the bone marrow was mixed with an equal volume of alpha-МЕМ (ICN) medium containing 0.2% methylcellulose (1500 cP, SigmaAldrich). After 40 min, most erythrocytes and granulocytes had precipitated, while the mononuclear cells remained in suspension. The suspended (upper) fraction was aspirated and centrifuged for 10 min at 450 g.
The cells from the sediment were resuspended in a standard cultivation medium that was composed of alphaMEM supplemented with 10% fetal bovine serum (Hyclone), 2 mМ L-glutamine (ICN), 100 U/ml penicillin (Ferein) and 50 mg/ml streptomycin (Ferein). The cells were cultured at 3 × 106 cells per T25 cm2 culture flask (Corning-Costar). When a confluent monolayer of cells had formed, they were washed with 0.02% EDTA (ICN) in a physiological solution (Sigma-Aldrich) and then trypsinized (ICN). The cells were seeded at 4 × 103 cells per cm2 of flask growth area. The cultures were maintained at 37˚C in 5% CO2. The number of harvested cells was counted directly; cell viability was checked by trypan blue dye exclusion staining.
All MMSC were immunophenotyped with the following markers using standard protocols: CD105, CD73, CD45, CD34, CD14 and HLA-DR. Antibodies were purchased from BD Pharmingen (CD105, CD59, CD73, CD90, CD31, CD34 and CD14), Sigma (CD45, FSP) and DAKO (HLA-DR).
For the CFU-F analysis, 106 and 5 × 105 mononuclear bone marrow cells were cultivated in T25 culture flasks in alpha-MEM supplemented with 20% fetal bovine serum (Hyclone), 2 mМ L-glutamine (ICN), 100 U/ml penicillin (Ferein) and 50 mg/ml streptomycin (Ferein). CFU-F concentration was measured on day 14 after staining with 4% crystal violet in 20% methanol.
2.2. RNA Isolation and Quantitative Reverse Transcriptase-Polymerase Chain Reaction
Total RNA was extracted from MMSC at passage 1 by the standard method [15], and cDNA was synthesized using the mixture of random hexamers and oligo (dT) primers. Gene expression levels were quantified by realtime quantitative PCR using hydrolysis probes (Taqman) and a Rotor-Gene 6000 machine (Corbett). Gene-specific primers were designed by the authors and synthesized by Syntol R&D. All primers and probes are provided in Supplement 1. The relative gene expression levels were determined by normalizing the expression of each target gene to the levels of BACT and GAPDH and calculated using the ΔΔCt method [16] for each MMSC sample.
2.3. Statistical Analysis
All values are presented as the means ± SEM. Data were analyzed using paired Student’s t-tests in Microsoft Excel. Correlations were estimated by the Spearman coefficient (R) in Statistica 6.0. P < 0.05 was considered significant.
3. RESULTS AND DISCUSSION
3.1. CFU-F Characteristics in Bone Marrow of Donors
Concentration of CFU-F in bone marrow of healthy donors declined with donors’ age (R = −0.68, p = 0.004), that agree with data of other investigators [17-19].
The whole group of donors was divided on the basis of age: group younger than median (32 years) was designated as “young”, and older than median as “old”. CFU-F concentration was significantly lower in the bone marrow of old, while it did not depend on the donors’ gender (Figure 1(a)).
The significant differences were revealed in relative expression level of genes markers of osteogenic osteopotin (secreted phosphoprotein 1, SPP1) and adipogenic peroxisome proliferator-activated receptor gamma (PPARG) differentiation that increased with donors age (Figure 1(b)). The expression levels of these genes strongly correlated with each other and with donors’ age (Table 1).
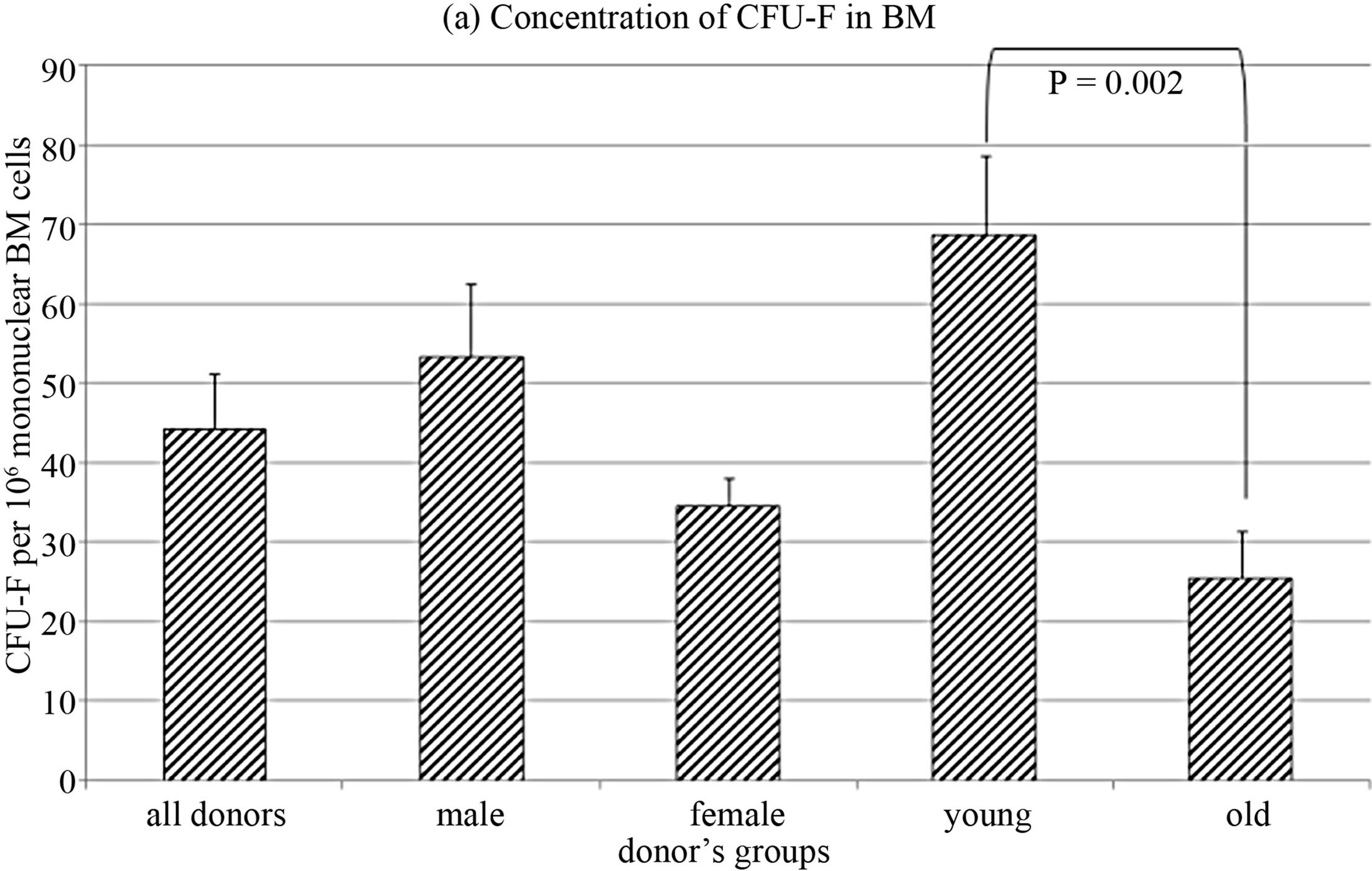
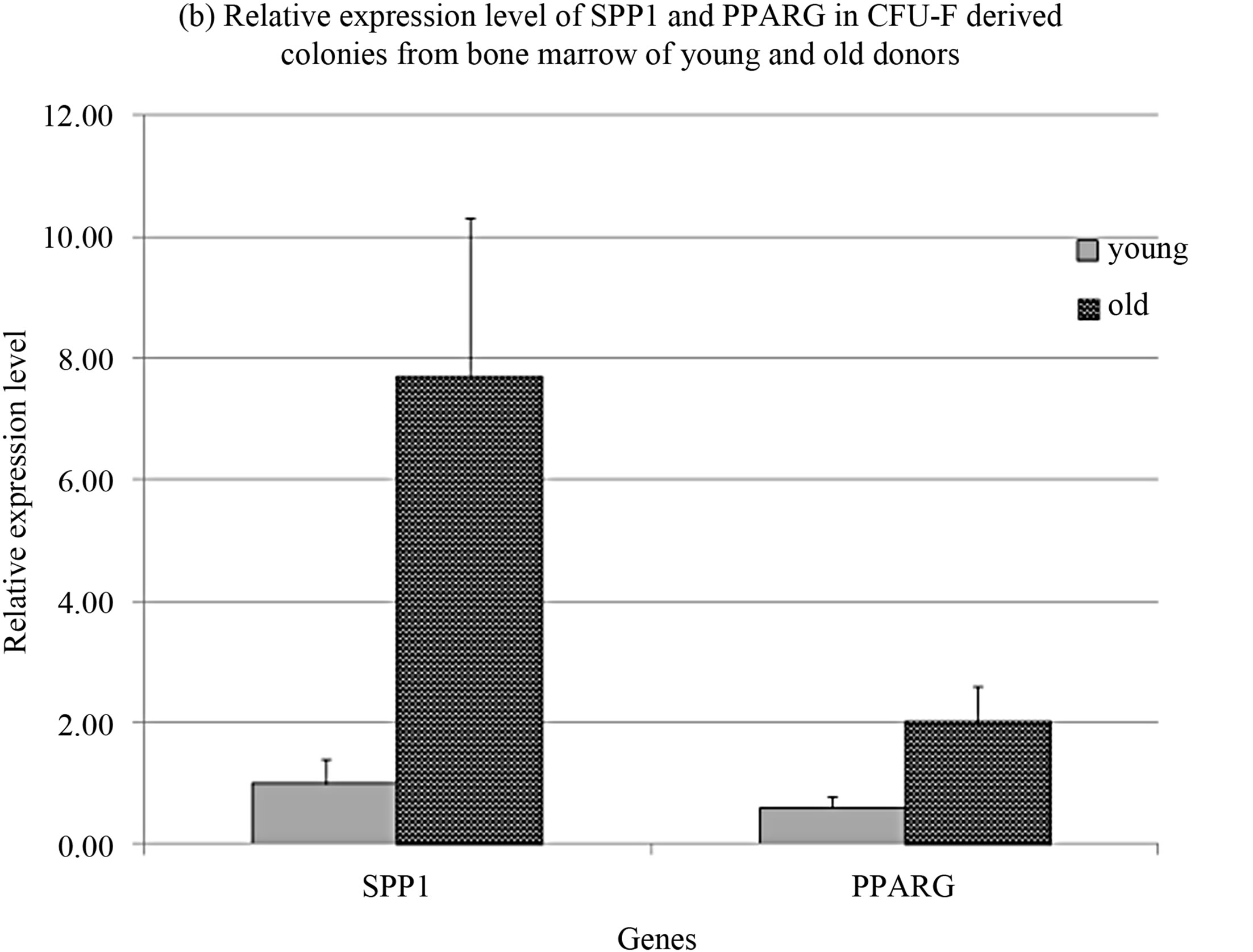
Figure 1. CFU-F characteristics in donors’ bone marrow.
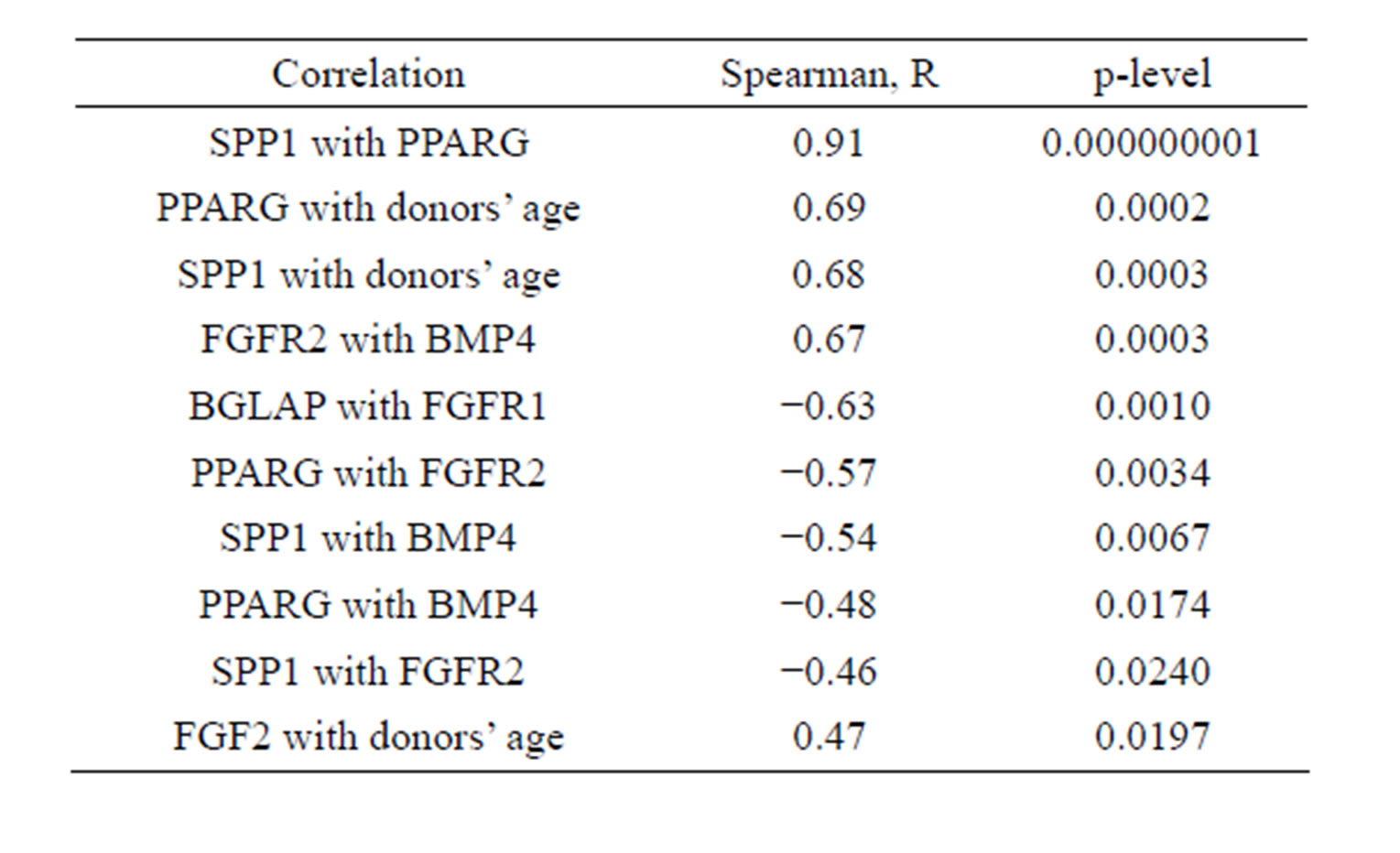
Table 1. Interconnections of CFU-F characteristics.
These data suggested the more differentiated status of CFU-F in old donors. Donors’ age also interconnected with relative expression level of basic fibroblast growth factor (FGF2) (Table 1).
Relative expression levels of several genes correlated with concentration of CFU-F in the bone marrow of donors independently of their age (Table 2).
Direct relationship between the expression of FGF2 and CFU-F concentration strongly agreed with indirect relationship of CFU-F concentration with FGF2 receptor 2 expression level.
All data demonstrated the heterogeneity of CFU-F from bone marrow of different donors.
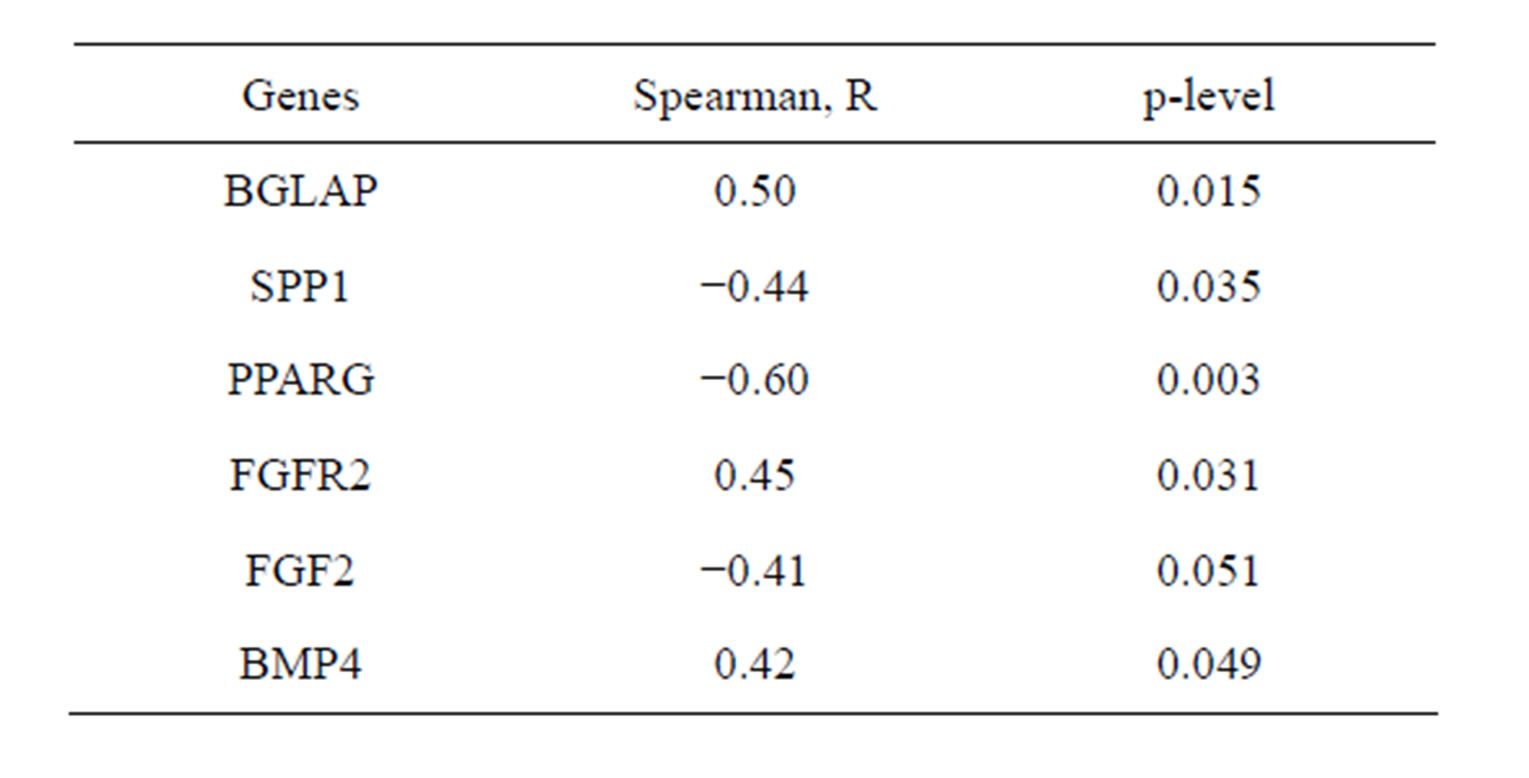
Table 2. Correlation of CFU-F concentration in donors’ bone marrow with relative expression levels of several genes in CFU-F derived colonies.
3.2. MMSC Characteristics
MMSC from the same samples of bone marrow of the donors whose CFU-F were studied in this work were immunophenotyped as described previously [20] and were shown to fit the standard for MMSC [7]. Cumulative MMSC production was analyzed for 5 passages. MMSC from donors were split to groups by the same criteria as CFU-F. No significant differences between groups were revealed (Figure 2(a)). However the distribution of cell production level between the groups strongly correlated with concentration of CFU-F in the same groups (R = 1, compare Figures 1(a) and 2(a)). Cumulative MMSC pro-
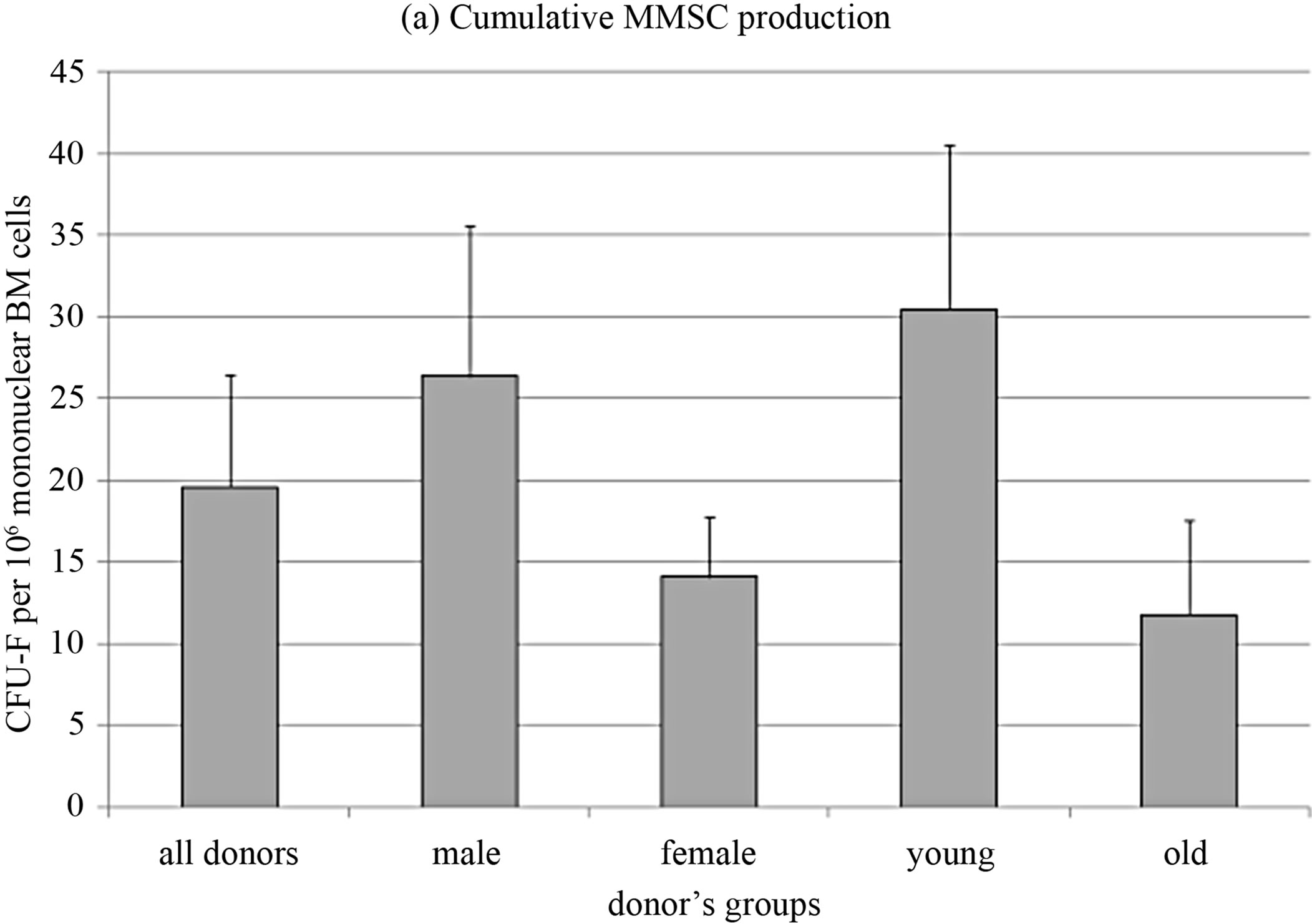
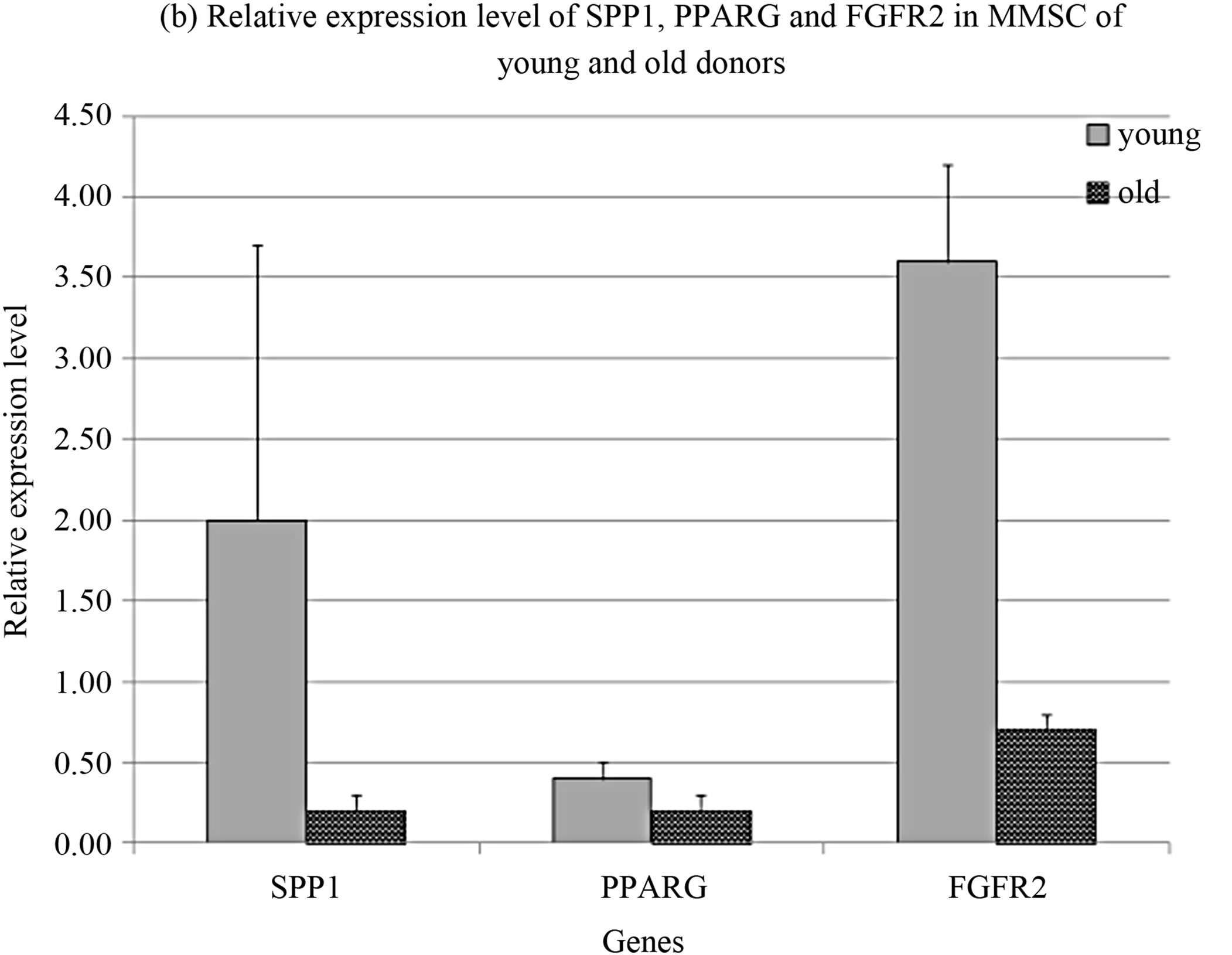
Figure 2. MMSC characteristics.
duction inversely correlated with donors age (R = −0.68, p = 0.0003) so as FGF receptor 2 expression level (R = −0.63, p = 0.007). Analogous data were introduced by Maijenburg with coauthors [21,22]. Inverse correlation was also observed in the expression level of SPP1 and FGF receptor 1 (R = −0.76, p = 0.0003) as well as SPP1 and the marker of chondrogenic differentiation SOX9 expression levels (R = −0.51, p = 0.036).
Comparison of MMSC from young and old donors revealed the decrease in expression levels of SPP1, PPARG and FGFR2 in old donors cells (Figure 2(b)).
FGF2 is the main growth factor for stromal cells [23]. It acts through receptors type 1 and type 2 [24,25]. The proliferative potential of MMSC correlated with the expression level of FGF receptors, that was reflected in the decrease of cumulative cell production and the expression level of FGFR2 with aging. SPP1 as osteogenic differentiation marker indicates the diminishing of MMSC proliferative potential accompanied by the decrease of FGFR1 expression level.
3.3. Comparison of MMSC and CFU-F Characteristics
Some authors consider MMSC and CFU-F to be identical [1,12]. Comparison of several genes’ expression level in MMSC and CFU-F derived colonies revealed significant differences between them (Figure 3). The differentiation markers SPP1 and PPARG were highly expressed in CFU-F colonies that prove their more differentiated status than MMSC in stromal stem cells hierarchy. The increased level of FGFR2, VEGF and BMP4 in MMSC pointed to their more immature nature.
This was accompanied with decreased expression of such differentiation markers as SPP1 and PPARG, but not SOX9.
The expression level of SOX9 in MMSC was significantly higher than in CFU-F colonies. It might be explained by the fact that chondrogenic differentiation is not typical for CFU-F unlike MMSC [26]. Nevertheless in the process of aging the expression level of SPP1, PPARG and FGFR2 decreased. It might underlay the reduction of osteogenesis and bone formation during aging [27]. The MMSC population is highly heterogenic [28], the cells differ by their proliferative potential and most part of the population is presented by mature nonproliferating cells [29]. The hierarchy of MMSC lineage commitment was initially described as a sequential loss of adipogenic and then chondrogenic potential to yield osteogenic progenitors [30,31]. Conversely, a subsequent study generated MMSC clones that exhibited adipogenesis but not chondrogenesis was published, suggesting that the hierarchical relationships may be more complex than originally proposed [10,32]. Our data demonstrated the predisposition of MMSC to chondrogenic lineage. This contradiction might be caused by the variation in proportion of cells biased to different lineages in MMSC from different donors.
On murine model it was shown that CFU-F are the progeny of MMSC [33].
The gene expression pattern in MMSC and CFU-F reflect the relationship between these 2 types of human stromal precursor cell. The expression level of differentiation markers inversely correlated with type 2 receptor to FGF2 in MMSC (Table 3), while concentration of CFUF in bone marrow correlates with FGFR1 expression in
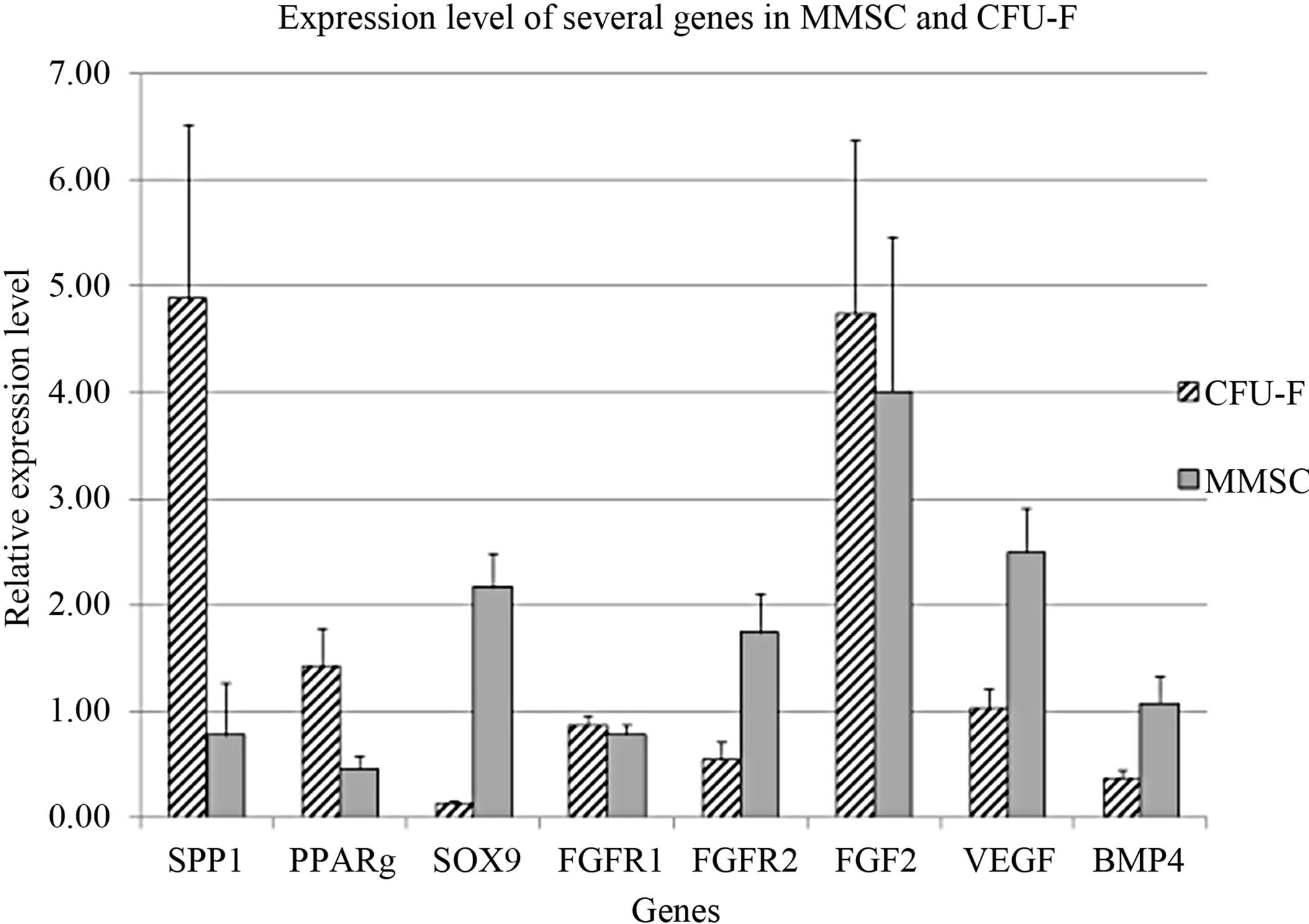
Figure 3. Comparison of gene expression in MMSC and CFU-F.
MMSC from the same bone marrow samples. Stromal precursor cells from individual bone marrow samples revealed the same differential tendency in both types of their progeny (MMSC and CFU-F). The existence of strong interrelationship between MMSC and CFU-F is also indicated by correlations between cumulative MMSC production with gene expression level in CFU-F (Table 4). The inverse correlation between SPP1 and PPARG expression level with MMSC production was shown. So more differentiated precursor cells have less ability to proliferate. Also the expression level of FGF2 in CFU-F derived colonies inversely correlated with cumulative MMSC production.
The CFU-F concentration in bone marrow of healthy donors varied from 0.33 to 118 per 106 mononuclear cells. In order to clarify the differences between stromal precursor cells in CFU-F poor and rich bone marrow samples, they were divided in 2 groups with more and less than median CFU-F concentration. The cumulative production of MMSC from bone marrow with low concentration of CFU-F was significantly lower than in the group with high CFU-F concentration (Figure 4(a)), that align with [14,34]. The expression levels of SPP1 and PPARG were significantly higher and BMP4 was significantly lower in MMSC of CFU-F poor group (Figure 4(b)).
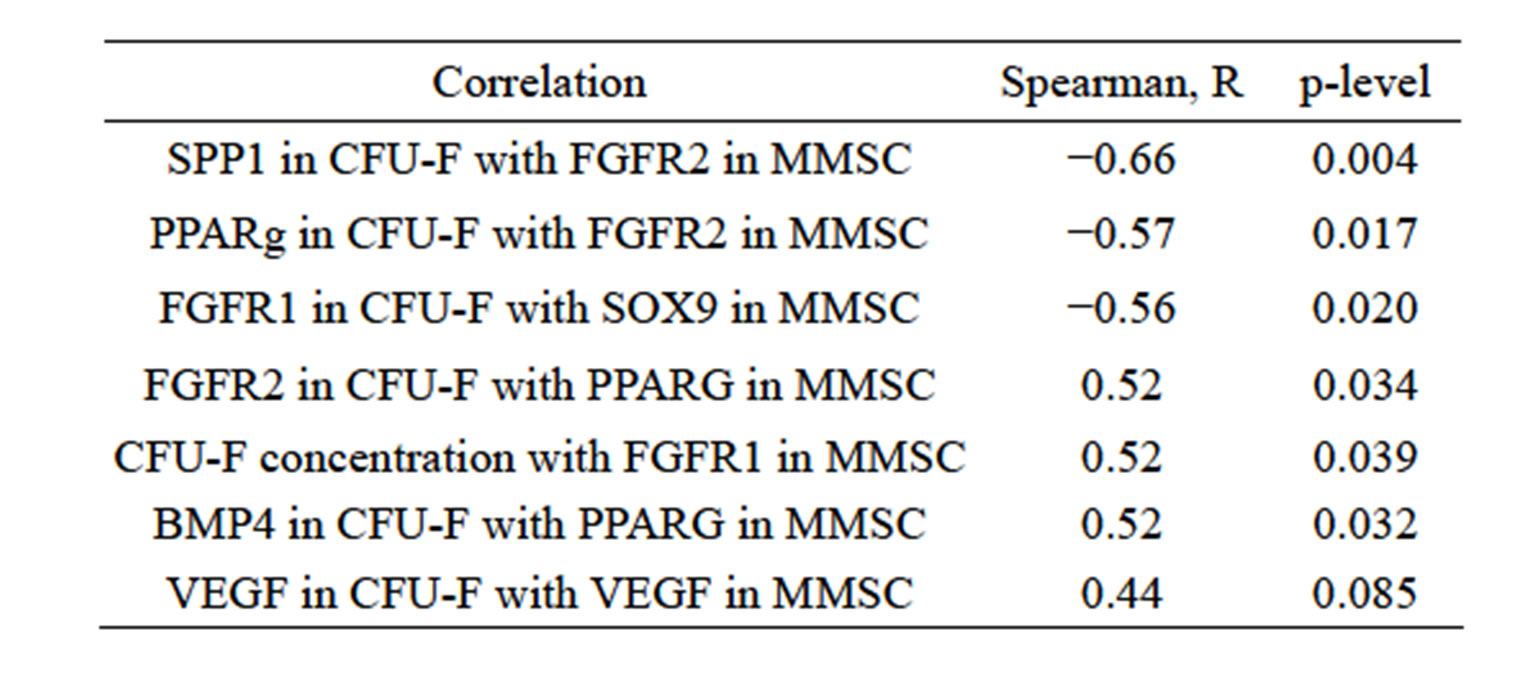
Table 3. Correlation of gene expression level in MMSC and CFU-F derived colonies.
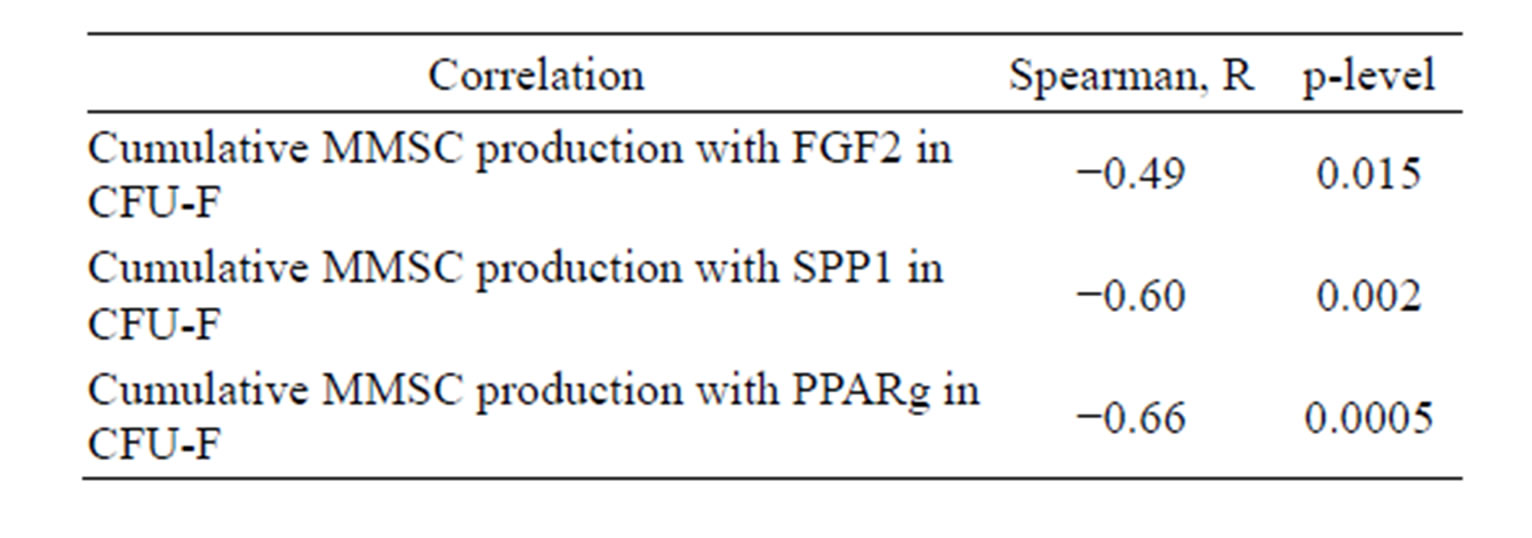
Table 4. Correlation of gene expression level in CFU-F derived colonies with cumulative MMSC production for 5 passages.
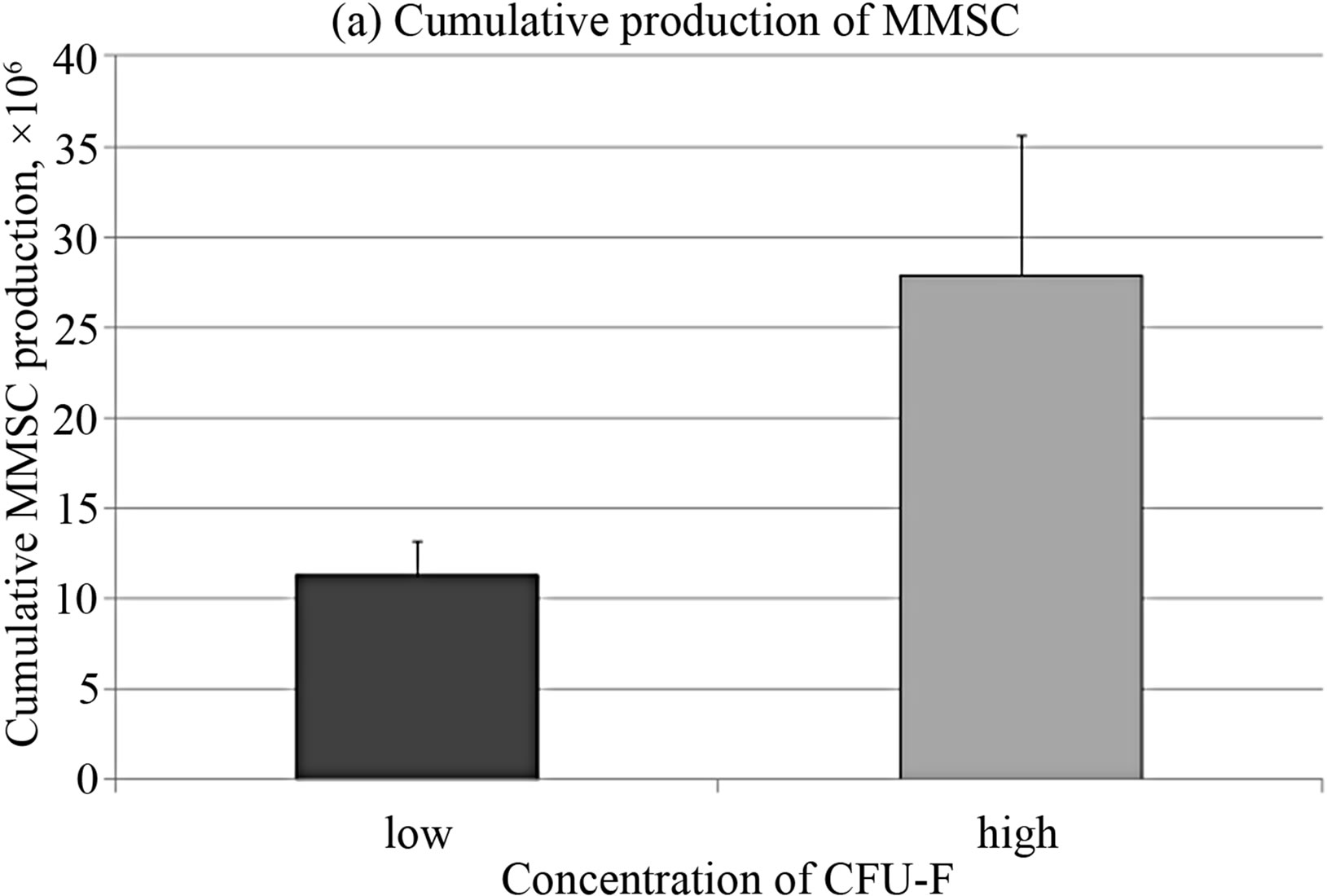
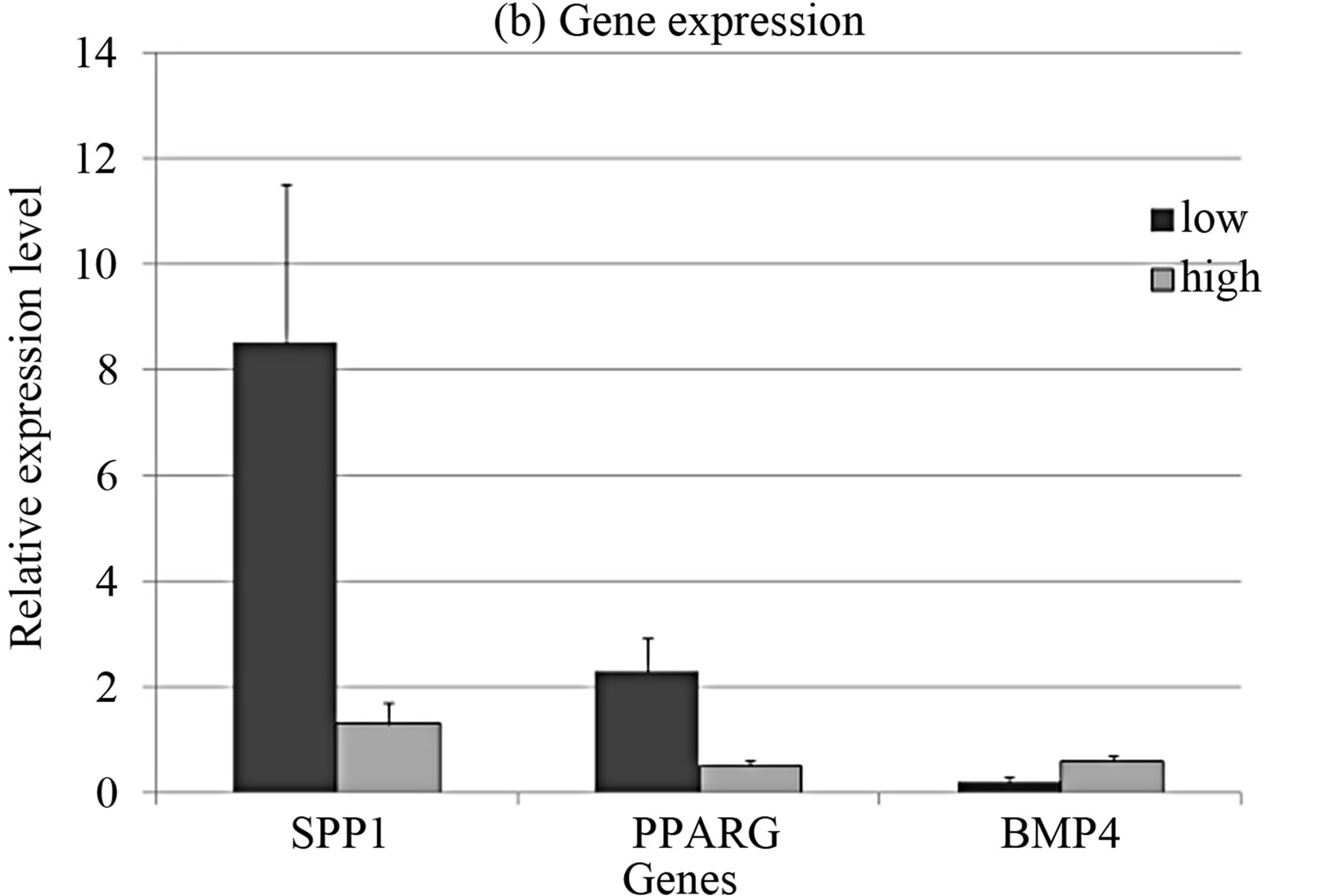
Figure 4. Cumulative cell production (a) and gene expression levels (b) in MMSC of two donors’ groups.
Gene expression analysis in CFU-F from the same groups demonstrated inversed pattern in SPP1 and PPARG expression (Figure 1(b)). This fact indicates that poor CFU-F number corresponds to more differentiated cells in the colonies.
4. CONCLUSION
The data obtained demonstrated the heterogeneity and hierarchical organization of both studied populations of stromal precursor cells MMSC and CFU-F. MMSC were presented by more immature cells than CFU-F and took up the higher position in hierarchical tree of mesenchymal stem cells. The differentiation rate and proliferative potential depended on the donors’ age in both MMSC and CFU-F populations. The proliferative potential, expression levels of several genes, especially differentiation markers, and ability to osteogenic differentiation significantly reduced with age. The organization of mesenchymal stem cells compartment needs further investigations.
5. ACKNOWLEDGEMENTS
The authors thank the staff of the Bone Marrow Transplantation Department. This study was supported by grants from the Russian Foundation for Basic Research (12-04-00457a and 13-04-00085a).
REFERENCES
- Caplan, A.I. (1991) Mesenchymal stem cells. Journal of orthopaedic research : Official publication of the Orthopaedic Research Society, 9, 641-650.
- Friedenstein, A.J., Gorskaja, J.F. and Kulagina, N.N. (1976) Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Experimental hematology, 4, 267-274.
- Prockop, D.J. (1997) Marrow stromal cells as stem cells for nonhematopoietic tissues. Science (New York, N.Y.), 276, 71-74. doi:10.1126/science.276.5309.71
- Short, B., Brouard, N., Occhiodoro-Scott, T., Ramakrishnan, A. and Simmons, P.J. (2003) Mesenchymal stem cells. Archives of Medical Research, 34, 565-571. doi:10.1016/j.arcmed.2003.09.007
- Beyer, N.N. and Da Silva Meirelles, L. (2006) Mesenchymal stem cells: Isolation, in vitro expansion and characterization. Handbook of Experimental Pharmacology, 174, 249-282. doi:10.1007/3-540-31265-X_11
- Horwitz, E.M., Le Blanc, K., Dominici, M., Mueller, I., Slaper-Cortenbach, I., Marini, F.C. et al. (2005) Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy, 7, 393-395. doi:10.1080/14653240500319234
- Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., Krause, D., et al. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy, 8, 315-317. doi:10.1080/14653240600855905
- Nifontova, I.N., Svinareva, D.A. and Drize, N.J. (2008) Stromal clonogenic precursors of hemopoietic microenvironment and their rank in the hierarchy of mesenchymal stem cells. Bulletin of Experimental Biology and Medicine, 145, 544-547. doi:10.1007/s10517-008-0137-z
- Tormin, A., Brune, J.C., Olsson, E., Valcich, J., Neuman, U., Olofsson, T., et al. (2009) Characterization of bone marrow-derived mesenchymal stromal cells (MSC) based on gene expression profiling of functionally defined MSC subsets. Cytotherapy, 11, 114-128. doi:10.1080/14653240802716590
- Russell, K.C., Phinney, D.G., Lacey, M.R., Barrilleaux, B.L., Meyertholen, K.E. and O’Connor, K.C. (2010) In vitro high-capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cells (Dayton, Ohio), 28, 788-798. doi:10.1002/stem.312
- Pevsner-Fischer, M., Levin, S. and Zipori, D. (2011) The origins of mesenchymal stromal cell heterogeneity. Stem Cell Reviews, 7, 560-568. doi:10.1007/s12015-011-9229-7
- Gao, J., Dennis, J.E., Muzic, R.F., Lundberg, M. and Caplan, A.I. (2001) The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells, Tissues, Organs, 169, 12-20. doi:10.1159/000047856
- Castro-Malaspina, H., Gay, R.E., Resnick, G., Kapoor, N., Meyers, P., Chiarieri, D., et al. (1980) Characterization of human bone marrow fibroblast colony-forming cells (CFUF) and their progeny. Blood, 56, 289-301.
- Cuthbert, R., Boxall, S.A., Tan. H.B., Giannoudis. P.V, McGonagle, D. and Jones, E. (2012) Single-platform quality control assay to quantify multipotential stromal cells in bone marrow aspirates prior to bulk manufacture or direct therapeutic use. Cytotherapy, 14, 431-440. doi:10.3109/14653249.2011.651533
- Chomczynski, P. and Sacchi, N. (1987) Single-step method of RNA isolation by acid guanidinium thiocyanatephenol-chloroform extraction. Analytical Biochemistry, 162, 156-159. doi:10.1016/0003-2697(87)90021-2
- Schmittgen, T.D. and Livak, K.J. (2008) Analyzing realtime PCR data by the comparative CT method. Nature Protocols, 3, 1101-1108. doi:10.1038/nprot.2008.73
- Galotto, M., Berisso, G., Delfino, L., Podesta, M., Ottaggio, L., Dallorso, S., et al. (1999) Stromal damage as consequence of high-dose chemo/radiotherapy in bone marrow transplant recipients. Experimental Hematology, 27, 1460-1466. doi:10.1016/S0301-472X(99)00076-4
- Gothard, D., Dawson, J.I. and Oreffo, R.O.C. (2013) Assessing the potential of colony morphology for dissecting the CFU-F population from human bone marrow stromal cells. Cell and Tissue Research, 352, 237-247. doi:10.1007/s00441-013-1564-3
- S. Kaneko, S. Motomura and H. Ibayashi (1982) Differentiation of human bone marrow-derived fibroblastoid colony forming cells (CFU-F) and their roles in haemopoiesis in vitro. British Journal of Haematology, 51, 217- 225. doi:10.1111/j.1365-2141.1982.tb02774.x
- Svinareva, D.A., Shipunova, I.N., Ol’shanskaia, I.V, Momotiuk, K.S., Drize, N.I. and Savchenko, V.G. (2010) The basic properties of mesenchymal stromal cells from the donor bone marrow: Superficial markers. Terapevticheskiĭ Arkhiv, 82, 52-56.
- Maijenburg, M.W., Kleijer, M., Vermeul, K., Mul, E., Alphen, F. Van, Schoot, C.E. Van Der, et al. (2011) The composition of the mesenchymal stromal cell compartment in human bone marrow changes during development and aging. doi:10.3324/haematol.2011.047753
- Maijenburg, M.W., Kleijer, M., Vermeul, K., Mul, E.P.J., Van Alphen, F.P.J., Van der Schoot, C.E. et al. (2012) The composition of the mesenchymal stromal cell compartment in human bone marrow changes during development and aging. Haematologica, 97, 179-183. doi:10.3324/haematol.2011.047753
- Gospodarowicz, D., Neufeld, G. and Schweigerer, L. (1987) Fibroblast growth factor: structural and biological properties. Journal of cellular physiology. Supplement, S5, 15-26.
- Neufeld, G. and Gospodarowicz, D. (1986) Basic and acidic fibroblast growth factors interact with the same cell surface receptors. The Journal of Biological Chemistry, 261, 5631-5637.
- Peters, K.G., Werner, S., Chen, G. and Williams, L.T. (1992) Two FGF receptor genes are differentially expressed in epithelial and mesenchymal tissues during limb formation and organogenesis in the mouse. Development (Cambridge, England), 114, 233-243.
- Kuznetsov, S.A., Friedenstein, A.J. and Robey, P.G. (1997) Factors required for bone marrow stromal fibroblast colony formation in vitro. British Journal of Haematology, 97, 561-570. doi:10.1046/j.1365-2141.1997.902904.x
- Stolzing, A., Jones, E., McGonagle, D. and Scutt, A. (2008) Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mechanisms of Ageing and Development, 129, 163-173. doi:10.1016/j.mad.2007.12.002
- Colter, D.C., Sekiya, I. and Prockop, D.J. (2001) Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proceedings of the National Academy of Sciences of the United States of America, 98, 7841- 7845. doi:10.1073/pnas.141221698
- Bigildeev, A.E., Zhironkina, O.A., Shipounova, I.N., Sats, N.V, Kotyashova, S.Y. and Drize, N.I. (2012) Clonal composition of human multipotent mesenchymal stromal cells. Experimental Hematology, 40, 847-856.e4. doi:10.1016/j.exphem.2012.06.006
- Muraglia, A., Cancedda, R. and Quarto, R. (2000) Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. Journal of Cell Science, 113, 1161-1166.
- Banfi, A., Muraglia, A., Dozin, B., Mastrogiacomo, M., Cancedda, R. and Quarto, R. (2000) Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Experimental Hematology, 28, 707-715. doi:10.1016/S0301-472X(00)00160-0
- Suva, D., Garavaglia, G., Menetrey, J., Chapuis, B., Hoffmeyer, P., Bernheim, L., et al. (2004) Non-hematopoietic human bone marrow contains long-lasting, pluripotential mesenchymal stem cells. Journal of Cellular Physiology, 198, 110-118. doi:10.1002/jcp.10396
- Nifontova, I., Svinareva, D., Petrova, T. and Drize, N. (2008) Sensitivity of mesenchymal stem cells and their progeny to medicines used for the treatment of hematoproliferative diseases. Acta Haematologica, 119, 98-103. doi:10.1159/000120440
- Hernigou, P., Poignard, A., Beaujean, F. and Rouard, H. (2005) Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. The Journal of Bone and Joint Surgery. American Volume, 87, 1430-1437. doi:10.2106/JBJS.D.02215

