Journal of Environmental Protection
Vol.09 No.06(2018), Article ID:85061,8 pages
10.4236/jep.2018.96043
Photocatalytic Removal of Organic Pollutants from Industrial Wastewater Using TiO2 Catalyst
H. K. Khalilova1*, S. A. Hasanova2, F. G. Aliyev2
1Institute of Physics of Azerbaijan National Academy of Sciences, Baku, Azerbaijan
2Azerbaijan Architecture and Construction University, Baku, Azerbaijan
Copyright © 2018 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: April 8, 2018; Accepted: May 28, 2018; Published: May 31, 2018
ABSTRACT
Degradation of organic pollutants in wastewater was investigated by photocatalysis method using anatase modification of titanium-dioxide (TiO2) catalyst with different structures. Laboratory experiments were carried out with industrial wastewaters consisting of different contents of organic pollutants. Two types of TiO2 catalyst: TiO2 having a size near to nanoparticles (about 3 - 4 micron in size) and the nanostructured 15 nm sized TiO2 were used to find optimum conditions of photocatalytic degradation and removal of organic compounds from wastewater. This paper discusses the effect of various parameters on the degradation rate including the catalyst concentration, process duration as well as the catalytic effectiveness of the TiO2 particles’ size. In addition, the effect of UV and visible light sources on the degradation process was studied. The process performed with nanostructured TiO2 catalyst under visible light was very successfully allowing best degradation of organic compounds.
Keywords:
Wastewater, Organic Compounds, Photocatalysis, Titanium-Dioxide, Chemical Oxygen Demand

1. Introduction
The environmental impact of industrial operations causes serious concern worldwide. Toxic organic compounds contribute significantly to the environmental pollution in industrially developed regions. They are the most priority pollutants because of their toxic effect on the ecosystem components and high migration ability. Like other industrial regions, the Absheron Peninsula of Azerbaijan is characterized with acute environmental situation. The previous studies of authors revealed high concentrations of toxic contaminants in soils, surface and ground waters in the Absheron territory, where about 70% of the country’s industrial potential is located [1] [2] . One of the problems associated with industrial activities is the huge amount of wastewater disposal to the environment. All the mentioned make important carrying-out researches to develop and apply advanced water treatment technologies capable of decontaminating hazardous pollutants.
A number of water treatment methods were used to remove chemical and microbial contaminants that pose threat to all the ecosystem components including human. However, low efficiency and formation of toxic by-products are main disadvantages of these processes. In the recent years, the effectiveness of water treatment processes has become more important due to the increase of environmental pollution, population growth and water resources demand. Photocatalysis is one of the methods which are so called advanced oxidation processes (AOP). AOP degrade the organic pollutants into harmless inorganic substances under moderate conditions. Photocatalysis is a process in which the catalyst changes the speed of a chemical reaction by the action of light. This was discovered in 1972 by Fujishima and Honda, who wanted to split water by the action of sunlight (photoelectrolysis), in analogy to photosynthesis process [3] [4] [5] [6] .
During photocatalysis, light energy is used to make photocatalyst active. After illumination by light with the appropriate wavelength, the photocatalyst induces the formation of strong oxidizing agents that decompose organic substances. Photocatalysis is an accelerator of the oxidation processes already active in nature. The substances used in photocatalysis to change the rate of chemical reactions, through the action of light, are semiconductors. Due to their electronic structure, which is characterized by a filled valence band (VB) and an empty conduction band (CB), semiconductors (metal oxides or sulfides) can act as sensitizers for light-induced redox processes [7] .
A heterogeneous photocatalytic system consists of semiconductor particles, which are in close contact with reaction medium. Exposing the catalyst to light excited states is generated, which is able to initiate subsequent processes like redox reactions and molecular transformations.
Among the various nanomaterials used for photocatalysis titanium dioxide―TiO2 is the most usual one for wastewater purification for its relatively low cost and high stability [8] [9] . TiO2 exists in three crystalline modifications: rutile, anatase, and brookite. Compared to rutile and brookite, anatase shows the highest photoactivity.
The photoactivity of TiO2 has been investigated extensively. TiO2 is a semiconductor with a band gap energy Eg = 3.2 eV. If this material is irradiated with photons of the energy > 3.2 eV (wavelength λ < 388 nm), the band gap is exceeded and an electron is promoted from the valence to the conduction band. Consequently, the primary process is the charge-carrier generation:
TiO2 + 2hν → 2 e− + 2 h+
H2O + 2h+→ 1/2O2+ 2H+
The event of the discovery of photocatalytic splitting of water on TiO2 electrodes marked the beginning of a new era in heterogeneous photocatalysis. Although TiO2 absorbs only approximately 5% of the solar light reaching the surface of the earth, it is the best investigated semiconductor in the field of chemical conversion and storage of solar energy. In recent years, semiconductor photocatalysis with TiO2 has been applied to important problems of environmental interest like degradation of organic compounds in wastewater. Nanoparticles of titanium dioxide were considered to be more efficient than bulk powder in photocatalytic field.
The purpose of our investigation was to study photocatalytic degradation of organic compounds in wastewater using crystalline TiO2 with various sizes.
2. Materials and Methods
Two types of anatase modification of TiO2 catalyst were used during the studies: TiO2 having a size near to nanoparticles, i.e. about 3 - 4 microns in size purchased from Russian CJSC “Vekton” (Figure 1) and the nanostructured 15 nm sized TiO2 obtained by hydrolysis of titanium tetraisopropoxide (TTIP) from Sigma Aldrich and subsequent precipitation (Figure 2).
Studies conducted earlier by specialists showed that the optimum value of pH is 3 - 3.5 for the degradation of hydrocarbons and other organic matter by TiO2 catalyst [10] [11] . We used 98% sulfuric acid from Sigma Aldrich to obtain pH = 3 medium.
The studies were carried out in laboratory conditions using industrial waters having different composition of organic contaminants. Initial laboratory tests
Figure 1. Electronic structure of TiO2 catalyst with a size 3 - 4 microns.
Figure 2. Size distribution of the TiO2 nanoparticles determined by means of a Brookhaven plus 90 nanosizer.
were conducted with oil industrial wastewater. The samples were collected from the territory of oil production enterprises in the vicinity of oil wells. Another part of experiments was addressed the study of the effect of nanostructured TiO2 catalyst on the reduction of organic compounds in olive oil mill water. The samples were collected in an olive mill wastewater stream.
The wastewater samples were diluted with respect to the original sample to operate with a more transparent polluted stream. To evaluate the degradation rate of organic compounds chemical oxygen demand (COD) was measured before and after each experiment. Analyses were carried out according to the known standard methods [12] .
Based on the data derived from experiments, a removal efficiency―R of organic contaminants was calculated according to the following formula [5] :
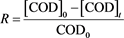
where, [COD]0 and [COD]t are the initial and at any irradiation time COD values.
3. Results and Discussion
The first series of experiments were carried out with oil industrial wastewater having an initial value of COD 8000 mg/l using TiO2 catalyst with a size near to nanoparticles.
The experiments were carried out in a cylindrical vessel with the capacity of about 500 ml located on the magnetic mixer to provide well-mixing of the catalyst and wastewater. A visible lamp of 300 watt in power was used as light source. The lamp was positioned at a 15 cm distance above wastewater to initiate the photocatalysis process. The experimental volume of the wastewater was 300 ml.
Preliminary experiments were conducted to study the effect of catalyst concentration on the process at 120 minute duration. The results are shown in Table 1.
As it can be seen from Table 1, the value of COD was reduced from 8000 to 5760 mg/l, by other words about 40% removal of organic matter was achieved using 0.5 g of TiO2 within 120 min. duration of photocatalysis.
The experiments have shown that further increase of the catalyst concentration was not resulted in significant change of COD value. Therefore, the next stage of studies was conducted to find the influence of photocatalysis duration on the degradation of organic compounds using the wastewater sample obtained from the last experiment, i.e. having COD of 5760 mg/l and at constant concentration of TiO2 catalyst (0.5 mg). The results are presented in Table 2.
The data in Table 2 shows that the increase of the process duration influences COD reduction to some extent. The value of COD was reduced from 5760 to 3200 within 360 minutes, i.e. 6 hours. Overall, no significant reduction was observed in COD reduction of oil industrial wastewater during the experiments. This can be attributed to either very high concentration of organic compounds in wastewater or to the low degradability of some hydrocarbons by TiO2 having particles of 3 - 4 microns in size. The results revealed that only 60% - 65% removal can be achieved with this type of TiO2 catalyst during 6 hours illumination. The calculated values of removal efficiency―R are illustrated in Figure 3.
Table 1. Effect of TiO2 concentration on COD reduction in oil idustrial wastewater.
Table 2. Effect of phocatalysis duration on COD reduction in oil idustrial wastewater.
Figure 3. Time dependence of the removal efficiency of organic pollutants from oil industrial wastewater using TiO2 catalyst.
Further experiments were carried out by a nanostructured titanium dioxide with a size of 15 nm. More performing configuration of the reactor was used in this case [11] . The reactor was a rectangular box with several partitions. The bottom of the box was covered by 20 g of glass spheres coated with nanoparticles. The reactor was placed in a bath maintained at a constant temperature of 20˚C. The system was provided with efficient air-gas contact to improve the degradation of organic compounds (Figure 4).
The glass spheres coated with TiO2 nanoparticles were put over a steel grid located at the bottom of the reactor and the air was fed at stable flow rate under the grid through a sprinkler consisting of a tube with a number of holes on the surface.
Two types of light source including an UV light lamp and a 50 watt visible light lamp were used in the experiments. The lamps were positioned over the reactor 10 cm far from the wastewater layer.
The obtained experimental data are reported in Table 3. Figure 5 illustrates the calculated values of the pollutants removal efficiency throughout the experimental runs with different light sources.
The results show very high reduction of COD in the presence of visible light, especially within 60 to 120 minutes of illumination. The use of TiO2 nanoparticles in photocatalytic process allows better adsorption of organic compounds on the catalyst surface facilitating their effective removal from wastewater.
4. Conclusion
The detoxification and removal of organic compounds in wastewater were investigated by photocatalysis method using two types of TiO2 catalyst. The laboratory experiments were mainly focused on the determination of the dependence of organic pollutants degradation on both the catalyst concentration and the process duration. The studies carried out with oil industry’s wastewater using TiO2 particles 3 - 4 microns in size demonstrated low degradation of organic compounds under 6 hours duration of photocatalytic process. The use of TiO2
Table 3. COD of olive mill wastewater for experiments performed by using UV lamp and visible light lamp.
Figure 4. Scheme of the reactor for organic pollutants removal from wastewater using TiO2 nanocatalyst.
Figure 5. Removal efficiency of organic pollutants by photocatalysis using TiO2 nanoparticles under irradiation of UV and visible light.
nanoparticles proved to be very efficient for the treatment of organic polluted wastewater by photocatalysis method. The experiments have revealed that the efficiency of photocatalytic degradation of organic compounds is also enhanced through applying visible light illumination and good air contact system. The results have shown that 99% removal of organic pollutants can be achieved with the use of TiO2 nanocatalyst within 120 min. Despite some expenditures associated with illumination, photocatalytic removal of organic pollutants from wastewater has considerable ecological and economical advantages over the conventional methods in long-term application.
Cite this paper
Khalilova, H.K., Hasanova, S.A. and Aliyev, F.G. (2018) Photocatalytic Removal of Organic Pollutants from Industrial Wastewater Using TiO2 Catalyst. Journal of Environmental Protection, 9, 691-698. https://doi.org/10.4236/jep.2018.96043
References
- 1. Aliyev, F.G. and Khalilova, H.Kh. (2014) The Anthropogenic Impact on Surface Water Resources in Azerbaijan. Energy and Environment, 25, 343-356. https://doi.org/10.1260/0958-305X.25.2.343
- 2. Khalilova, H.Kh. and Mammadov, V.A. (2014) The Assessment of Anthropogenic Impact on Heavy Metal Pollution of Soils and Sediments in Urban Areas of Oil Industrial Region of Azerbaijan. Polish Journal of Environmental Studies, 25, 159-166. https://doi.org/10.15244/pjoes/60723
- 3. Inamdar, J. and Singh, S.K. (2008) Techno-Economic Analysis of Zero Effluent Discharge by Use of Solar Detoxification at Household Level. International Journal of Natural Sciences and Engineering, 1, 208-211.
- 4. Kositzi, M., Poulios, I., Malato, S., Caceres, J. and Campos, A. (2004) Solar Photocatalytic Treatment of Synthetic Municipal Wastewater. Water Research, 38, 1147-1154. https://doi.org/10.1016/j.watres.2003.11.024
- 5. Shahrezaei, F., Akhbari, A. and Rostami, A. (2012) Photodegradation and Removal of Phenol and Phenolic Derivatives from Petroleum Refinery Wastewater Using Nanoparticles of TiO2. Energy and Environment, 3, 267-274.
- 6. Fujishima, A. and Honda, K. (1972) Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature, 238, 37-38 https://doi.org/10.1038/238037a0
- 7. Burigo, E. (2014) Photocatalytic Degradation of Petroleum Hydrocarbons: Lab Scale Tests on Contaminated Soil Samples. Master Thesis, Universita Degli Studi Di Padova, 91 p.
- 8. Tu, W., Lin, Y.-P. and Bai, R. (2013) Removal of Phenol in Aqueous Solutions by Novel Buoyant Composite Photocatalysts and the Kinetics. Separation and Purification Technology, 115, 180-189. https://doi.org/10.1016/j.seppur.2013.05.009
- 9. Hashimoto, K., Irie, H. and Fujishima, A. (2005) TiO2 Photocatalysis: A Historical Overview and Future Prospects. Japanese Journal of Applied Physics, 44, 8269-8285. https://doi.org/10.1143/JJAP.44.8269
- 10. Okamoto, K., Yamamoto, Y., Tanaka, H., et al. (1985) Heterogeneous Photocatalytic Decomposition of Phenol over TiO2 Powder. Bulletin of the Chemical Society of Japan, 58, 2015-2022. https://doi.org/10.1246/bcsj.58.2015
- 11. Ibrahimova, S., Aliyev, F.G., Stoller, M. and Chanese, A. (2016) Optimal Configuration of a Photocatalytic Lab-Reactor by Using Immobilized Nanostructured TiO2. Chemical Engineering Transactions, 47, 199-204.
- 12. Standard Methods for Examination of Water and Wastewater, American Public Health Association/American Water Works Association/Water Environment Federation, 19th Edition 1995, Washington DC.







