International Journal of Organic Chemistry
Vol.3 No.4(2013), Article ID:41376,5 pages DOI:10.4236/ijoc.2013.34036
New Method of Palladium Metal Trapping through Resins in Antiviral Drug: Valacyclovir HCl
1Research and Development, Integrated Product Development, Innovation Plaza, Dr. Reddy’s Laboratories Ltd., Bachupally, Quthubullapur, India
2Dr. MACS Bio-Pharma Pvt. Ltd., Western Hills, Kukatpally, Hyderabad, India
3Department of Chemistry, Institute of Science and Technology, J. N. T. University, Kukatpally, Hyderabad, India
Email: *drmac_s@yahoo.com
Copyright © 2013 Keshava Navin Kumar Reddy et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received October 4, 2013; revised November 8, 2013; accepted November 23, 2013
Keywords: Valacyclovir Hydrochloride; Antiviral Drug; N-Phthalimide-L-Valine Ester; Monomethylamine; Commercially Scalable Process; Less Cycle Time
ABSTRACT
This process describes a novel technique for effective trapping of “Pd” metal through resins during the process development of L-valine, 2-[(2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl) methoxy] ethyl ester, and mono hydrochloride known as a Valacyclovir hydrochloride (1). This technique is suitable for large-scale production of 1 and it is described here Pd metal trapping by using different resins.
1. Introduction
Valacyclovir (Valtrex®) (Figure 1) is an orally active prodrug of the antiviral drug acyclovir [1,2]. Valacyclovir is the L-Valyl ester of the antiviral drug acyclovir which is active against herpes simplex virus types 1 and 2 and varicella zoster virus [3-5]. After oral administration, the prodrug Valacyclovir is rapidly absorbed from the gastrointestinal tract and nearly completely converted to acyclovir and L-valine. Acyclovir is a specific and selective inhibitor of viral herpes replication and is neither highly lipid nor aqueous soluble, with limited and variable oral bioavailability (15% - 21%) that decreases with increasing doses. Conversely, Valacyclovir has an oral bioavailability of 3 to 5 times higher than that of Acyclovir itself [6,7].
Synthesis of 1 involved streglich esterification (Scheme 1) between 9-(2-hydroxyethoxy) methyl)-2-amino- 1H-purin-6-(9H)-one 4 and N-Benzyloxy carbonyl-Lvaline 3 in the presence of N, N-(dimethylamino) pyridine (DMAP) and a coupling reagent dicyclohexylcarbodiimide (DCC) in dimethylformamide (DMF) medium

Figure 1. Structural framework of Valacyclovir hydrochloride (1).
obtain N-benzyloxy protected ester 2. This is the further deprotection in the presence of Pd/C catalyst to afford 1 [8].
Reported reference process suffers from a major disadvantage of metal content, which is coming higher level, which is not acceptable by International Conference on Harmonization (ICH) and the metal content should be less than 10 ppm. [Pd] This metal is carried from the Pd/C catalyst which is used for deprotection of 2, which makes process less viable for commercial production.
Pd Adverse Effects
Metal cations in dental alloys such as mercury and palladium are continuously released and accumulated in the kidneys, liver, thyroid, brain. Mercury and palladium have high levels of galvanic current densities when near other metals, with the current densities of Pd alloys approx. It is 10 times higher than for high noble alloys. This causes extensive migration of mercury and palladium to saliva, tooth roots, jaw, gums, and other parts of the body [9,10].
Palladium is cytotoxic and kills or damages cells. Palladium also causes considerable damage and degradation of DNA and exacerbates hydroxyl radical damage [11- 13]. Palladium also damages cell mitochondria and inhibits enzyme activity and function [14-16]. Palladium also causes significant numbers of allergic reactions as well as contacts dermatitis, stomatitis, lichinoid reactions, and periodontal gum disease [17-21]. Herein we report a simple and effective resin absorption method for Pd metal and it is consistently showing less than 5 ppm level to produce 1.
2. Results and Discussion
Our efforts are to develop a robust process for desired product 1 to get metal content below 10 ppm described below in greater level with different resins for effective trapping of “Pd” metal.
During the isolation of 1 an in-depth study was done by treating the reaction mass with different reagents like thiourea, ethylene di amine tetra acetate (EDTA), Zeolites, CH-97 resin, and T-63 resin. The metal trapping was not so effective by using thiourea, EDTA, and Zeolites. Out of all these CH-97 resin & T-63 resin are effectively trapping comparatively T-63 resin more effective and yield loss also less. So decided T-63 resin is suitable commercialization of process. “Pd” metal was selectively trapped by using T-63 resin. It is made up with resin polystyrene co-polymer and macroporoces with high porosity and large surface area.
In our approach process involves (Scheme 2) deprotection of 2 by using 10% Pd/C in dimethylformamide medium and during the workup Pd/C catalyst removed by filtration and further concentration of filtrate and followed by pH adjustment up to 4.0 - 4.5 with 36% Aq. HCl. Finally treating with T-63 Resin 5 (Figure 2) further solid was isolated by adding anti solvent acetone to the filtrate to afford 1.
Attempts made by using different resins effective trapping of “Pd” metal during the isolation of 1 described below in greater details in Table 1.
Above results of “Pd” content with different resins are mentioned in graphical diagram Figure 3.
3. Conclusion
In summary, the present study describes a novel technique for effective trapping of “Pd” metal through resins during the process development of L-valine, 2-[(2-amino-1, 6-dihydro-6-oxo-9H-purin-9-yl) methoxy] ethyl ester, and mono hydrochloride known as a Valacyclovir hydrochloride (1). This technique is suitable for large-scale production of 1 and it is described here “Pd” metal trapping by using different resins.
4. Experimental Section
4.1. Reagents & Condition
Warm the 4 & DMF mixture at 60˚C, to that add 3 and DMAP & DCC. Maintain for 24 h at 25˚C - 35˚C. Stir for 48 h at 25˚C - 35˚C after addition of same quantity of 3 and DMAP & DCC to the above reaction mixture, re-

Scheme 1. Synthetic scheme.
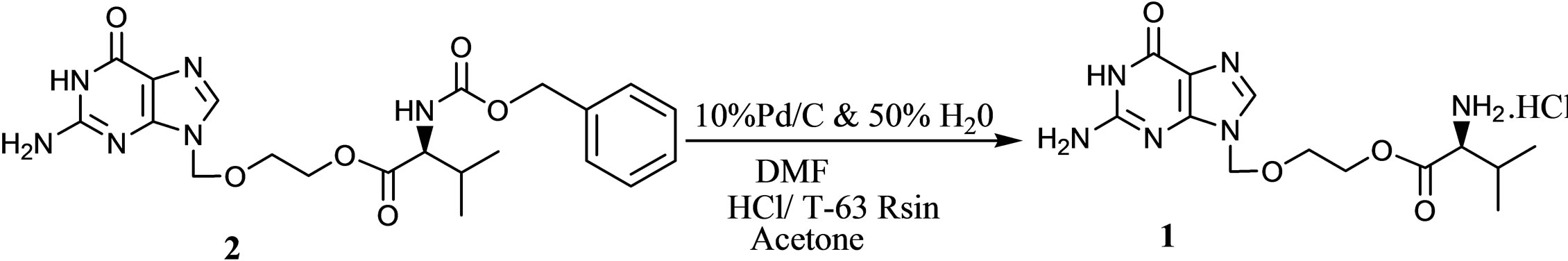
Scheme 2. Synthetic scheme.

Table 1. Pd content results after treating with different resins.

Figure 2. Structural framework of T-63 resin (5).

Figure 3. “Pd” content with different resins.
move the undissolved by filtration and further concentrate the filtrate to obtain 2. A mixture of 2 and 5% palladium on carbon catalyst-50% water and DMF were taken into par apparatus and maintained for 3 - 4 h and finally recrystalized in water/ethanol mixture (1:3 v/v) yield 1.
A mixture of 2, 10% palladium on carbon catalyst- 50% water and DMF take in to par apparatus maintain for 3 - 4 h and further converted in to Hydrochloride by using HCl and followed by resin treatment (T-63 Resin) and finally isolation with acetone medium yields 1.
4.1.1. Synthesis of Val Acyclovir Hydro Chloride (1): (Reported Process)
Compound 2 of 5.0 g (0.01 mol), 5% palladium on carbon catalyst-50% water (2 g), and DMF (50 mL) were taken into par apparatus applied 4.0 kg/cm2 hydrogen pressures and maintained for 3 h. Filter the catalyst and concentrate the filtrate to give oil. Obtained oil was crystallized from water/ethanol (1:3 v/v) yields 1.5 g of 1.
4.1.2. Synthesis of Val Acyclovir Hydro Chloride (1): (Improved Process)
Compound 2 of (50 g, 0.1 mol), 10% palladium on carbon catalyst-50% water (5.0 g) and DMF (500 mL) were taken into par apparatus applied 4.0 kg/cm2 hydrogen pressures and maintained for 3 - 4 h. Filter the catalyst and wash with DMF (100 mL). Further concentrate the filtrate up to minimum volume obtain, cool the residue to 5˚C - 10˚C. Add water (125 mL). Adjust the pH up to 4.0 - 4.5 with 36% HCl (9.3 mL). Filter through Hyflow and wash with water (50 mL). Add T-63 resin 5 (1.4 g) to the filtrate and stir for 30 - 60 min. Filter through a 0.45 μm. Add acetone (375 mL) obtain filtrate and stir for 1 - 2 h. A product was isolated by filtration and washed with acetone (50 mL), and dry the product at 60˚C - 70˚C yields 1.
4.1.3. Estimation of Pd Content in Valcyclovir Hydrochloride 1
The 1HNMR spectra were measured in CDCl3 and DMSO-d6, using 200 and 50 MHz, respectively on a Varian Gemini 200 MHz FT NMR spectrometer. The chemical shifts are reported in δ ppm relative to TMS. FT-IR spectra were recorded in the solid state as a KBr dispersion using a perkin-Elmer 16428 FT-IR spectrophotometer. The mass spectrums were obtained using a shimadzu QP-8000a mass spectrometer with an electron energy set to 1.5 kV. The sample was introduced via the mass inlet using a LC pump (LC 10ADVP series) and an auto l injector (SIL 10ADVP). The heat block crossed dissolution line temperature at 230˚C and 250˚C respectively. Typical AAS conditions and furnace programme in AAS in the estimation of Pd content were presented in Tables 2 & 3. The Pd content observed 30 ppm in the reported process and 2.54 ppm in the present improved process using AAS (Table 4).
4.2. Preparation of Blank
Transfer 4.62 mL of 65% Suprapur Nitric acid (merk grade) into a 1000 mL flask and make up to the mark

Table 2. Typical AAS conditions in the estimation of Pd content.

Table 3. Furnace programme in AAS in the estimation of Pd content.
*Sample injection volume: 20 µl.

Table 4. Estimation of Pd content measured by atomic absorption spectroscopy.
with Milli Q Water. This gives the concentration of 0.3% Nitric acid solution and it is used as the blank.
4.3. Preparation of Sample
Weigh accurately about 200 mg of Valacyclovir sample into a 100 ml Vol. Flask dissolves and dilutes to the volume with 0.3% Nitric acid solution; sonicate this solution until the sample is totally dissolved. Then add the samples in the auto sampler vials for the estimation of Palladium by furnace.
5. Acknowledgements
We thank the management of R & D-IPDO, Dr. Reddy’s Laboratories Ltd., for supporting this work. Cooperation from the colleagues from the analytical research and development is highly appreciated.
REFERENCES
- L. M. Beauchamp, G. F. Orr, P. de Miranda, T. Brunette and T. A. Krenitsky, “Amino Acid Ester Prodrugs of Acyclovir,” Antiviral Chemistry and Chemotherapy, Vol. 3, No. 3, 1992, pp. 157-164.
- R. J. Crooks and A. Murray, Antiviral Chemistry & Chemotherapy, Vol. 5, No. S1, 1994, pp. 31-37.
- C. M. Perry and D. Faulds, “Valacyclovir,” Drugs, Vol. 52, No. 5, 1996, pp. 754-772. http://dx.doi.org/10.2165/00003495-199652050-00009
- L. H. Wang, M. Schultz, S. Weller, M. L. Smiley and M. R. Blu, “Valacyclovir Compared with Acyclovir for Improved Therapy for Herpes Zoster in Immunocompetent Adults,” Antimicrobial Agents and Chemotherapy, Vol. 39, No. 7, 1995, pp. 1546-1553. http://dx.doi.org/10.1128/AAC.39.7.1546
- D.-K. Kim, N. Lee, G. J. Im, H.-T. Kim and H. K. Key, “Synthesis and Evaluation of 2-Amino-6-fluoro-9-(2-hydroxyethoxymethyl) Purine Esters as Potential Prodrugs of Acyclovir,” Bioorganic & Medicinal Chemistry, Vol. 6, 1998, pp. 2525-2530. http://dx.doi.org/10.1016/S0968-0896(98)80026-6
- A. S. Jadhav, D. B. Pathare and M. S. Shingare, “Development and Validation of Enantioselective High Performance Liquid Chromatographic Method for Valacyclovir, an Antiviral Drug in drug Substance,” Journal of Pharmaceutical and Biomedical Analysis, Vol. 43, No. 4, 2007, pp. 1568-1572. http://dx.doi.org/10.1016/j.jpba.2006.11.018
- J. Soul-Lawton, E. Seaber, N. On, R. Wootton, P. Rolan and J. Posner, “Absolute Bioavailability and Metabolic Disposition of Valacyclovir, the L-valyl Ester of Acyclovir, Following Oral Administration to Humans,” Antimicrobial Agents and Chemotherapy, Vol. 39, No. 12, 1995, p. 2759.
- L. M. Beauchamp and N. C. Raleigh, “Synthesis of Amino Acid Esters and Pharmaceutical Acceptable Salts and Treatment of Viral Infections,” US Patent No. 4957924.
- W. P. Bieger, et al., “Immunotoxocolgy of Metals,” Zur Deutshcen Auszahe, 1996.
- K. Bonnig, et al., “Quantitative Analysis of the Corrosion rates of Palladium Alloys,” Dtsche Azhnarztliche Zeitschrift, Vol. 45, No. 8, 1990, pp. 508-510.
- Y. Kawata, et al., “Cytotoxicity of Pd-Co Dental Alloys,” Journal of Dental Research, Vol. 60, No. 8, 1981, pp. 1403-1409. http://dx.doi.org/10.1177/00220345810600080301
- T. Z. Liu, et al., “Palladium Exacerbates Hydroxyl Radical Mediated DNA Damage,” Free Radical Biology and Medicine, Vol. 23, No. 1, 1997, pp. 155-161. http://dx.doi.org/10.1016/S0891-5849(96)00553-9
- C. K. Pillai, et al., “Interaction of Palladium with DNA,” Biochimica et Biophysica Acta, Vol. 474, No. 1, 1977, pp. 11-16. http://dx.doi.org/10.1016/0005-2787(77)90209-X
- G. M. Kolesova, et al., “Effect of Palladium Compounds on Mitrochondrial Enzymatic Systems,” Voprosy Meditsinskoi Khimii, Vol. 25, No. 5, 1979, pp. 537-540.
- J. D. Spikes, et al., “Enzyme Inhibition by Palladium,” Biochemical and Biophysical Research Communications, Vol. 35, No. 3, 1969, pp. 420-422. http://dx.doi.org/10.1016/0006-291X(69)90516-6
- M. D. Shultz, et al., “Palladium—A New Inhibitor of Cellulase Enzyme Activity,” Biochemical and Biophysical Research Communications, Vol. 209, No. 3, 1995, pp. 1046-1052. http://dx.doi.org/10.1006/bbrc.1995.1603
- A. M. Al-Roubaie, “Condition of the Periodontion of Teeth with Silver-Palladium Bridge,” Fogorvosi Szemle, Vol. 79, No. 7, 1986, pp. 207-212.
- D. Downey, “Contact Mucositis Due to Palladium,” Contact Dermatitis, Vol. 21, No. 1, 1989, p. 54. http://dx.doi.org/10.1111/j.1600-0536.1989.tb04688.x
- J. A. Marcusson, “Contact Allergies to Palladium Chloride,” Contact Dermatitis, Vol. 34, No. 5, 1996, pp. 320- 323. http://dx.doi.org/10.1111/j.1600-0536.1996.tb02215.x
- J. Vilaplana, C. Romaguera and F. Cornellana, “Contact Dermatitis and Adverse Oral Mucous Membrane Reactions Related to the Use of Dental Prostheses,” Contact Dermatitis, Vol. 30, No. 2, 1994, pp. 80-84.
- A. Henston-Pettersen, “Casting Alloy Side Effects,” Advances in Dental Research, Vol. 6, No. 1, 1992, pp. 38- 43.
NOTES
*Corresponding author.

