Paper Menu >>
Journal Menu >>
 Advances in Ma terials Physics and Che mist ry, 2012, 2, 16-20 doi:10.4236/ampc.2012.24B005 Published Online December 2012 (htt p://www.SciRP.org/journal/ampc) Copyright © 2012 SciRes. AMPC Sol-Gel Synthesis of TiO2 Thin Films from In-House Nano-TiO2 Powder Mohd Zainizan Sahdan1, Nafarizal Nayan1, Samsul Haimi Dahlan1, Mahdi Ezwan Mahmoud2, Uda Hashim3 1Microelectroni c and Nanotechnology-Shamsuddi n Research Cen tre (MiNT-SRC), Faculty of Electrical and El ectronic Engi ne e r ing , Universiti Tun Hussein Onn Malaysia, Batu Pahat, Johor, Malaysia 2Material Technology Group, Nuclear Agency of Malaysia, 43000 Kajang, Selangor, Malaysia 3Institute of Nano Electronic Engineering (INEE), Universiti Malaysia Perlis, Arau, Perlis, Malaysia Email: zainizno@gmail.com Received 2012 ABSTRACT This paper presents the optimization process in sol-gel technique to synthesize Titanium dioxide (TiO2) thin films using in-house Nano-TiO2 powder. Nano-TiO2 powder was previously synthesized in our lab from ilmenite which is a tin mining byproduct using a modified hydrothermal method. By varying the mass of Nano-TiO2 powder and acetic acid (catalyst) concentration in the sol-gel process, highly transparent TiO2 thin films were obtained . The thin films were character ized by field ef fect scanning elect ron micro- scope (FESEM), atomic force microscopy (AFM), thickness profiler, ultra-violet-visible sp ectrometer (UV-Vis) and curr ent-voltage (I-V) measure ment syste m. This p aper also d emonstrates th e TiO2 thin films are sensitive towards isopropanol (IPA) solution where the I-V response of the thin films changed sharply as IPA was dropped onto the thin film’s surface. The electrical property shows the thin film has potential applications for chemical sen s ors and solar cells . Keywords: Itanium Dioxide; Ilmenite; Sol-gel; Tin mining 1. Introduction Tianium dioxide (TiO2) or known as titania has been reported widely for its numerous applications from optoelectronics to cosmetics [1-3]. TiO2 has excellent photocatalytic oxidative properties that depend on the crystallinity and crystal form [4]. Due to the photocatalytic activity, TiO2 has been used in water and air pollution treatments [5]. It also exhibits unique electrical and chemical properties that can be utilized in various techno- logical and engineering applications such as humidity sensor, gas sensor and membrane [6,7] . In addition, TiO2 is also pro- posed for solar cells and laser diodes for its high refractive index and stability [8]. Although the starting material of TiO2 powder can be obtained easily in the market, the price is quite expen- sive especial ly for resear ch purposes i n Malaysia. Th erefore, an alternative way of using in-house nano-TiO2 powder (anatase) synthes ized from Ilmenite powder (from Malaysian Tin mining waste), is propo sed. Using this in-house nano-TiO2 powder, the cost o f the starti ng material can be reduced up to 80%. The problem of using nano-TiO2 powder is the low solubility in organic solvent such as ethanol and isopropanol. Therefore, optimization on the mass of the starting material and catalyst is required. Sol-gel process is proposed since it is a convenient and versatile method for preparing transparent thin film at low temperature [9]. The sol-gel process involved many complex processes for both chemical and structural nature. Before gel formation (polymerization), two stages are indentified: i) hy- drolysis of the organometallic group precursor, and ii) poly- condensation. The physical, chemical and mechanical proper- ties are much dependant on the properties of the precursor solu- tion [10]. Therefore, optimizing the precursor solution may produce great results of TiO2 thin film. Sol-gel process is very convenient to deposit transparent materials in combination with spin coating technique. The resulting coatings are of high purity and structural homogeneity depending on the parameters opti- mization. 2. Experimental Indium Tin Oxide (ITO) was used as the substrate which has dimension of 1.5 cm x 1.5 cm. The su bstrat e was cleaned usin g acetone in ultrasonic bath for 5 minutes at 50ºC. Then, it was blown dry with nitrogen gas. Different TiO2 solution was prepared using different mass of nano-TiO2 powder which is 1g, 0.4 g, 0.1 g and 0.05 g. Each powder will be stirred in 30 ml of ethanol mixed with 6 ml of acetic acid. After underwent ageing process for 20 hours, the solution was spin coated onto the ITO substrate for 10 layers. The deposition was using 2-steps spin coating (1000 r.p.m. for 30 s and 3000 r.p.m. for 60 s). Every layer was preheated at 100ºC for 3 minutes. The thin films were annealed at 500ºC for 1 hour to improve the structural property. Again, after underwent slow cooling at room temperature, the thin films were charact er ized to find the optim um Nano-TiO2 mass. The acetic acid concentration was optimized using different acetic acid volumes which are 0 ml, 6 ml, 10 ml and 30 ml. It was mixed with nano-TiO 2 powder using the optimum mass in the previous experiment. It was stirred in 30 ml of ethanol for 20 hours. Using the same spin coater step, the TiO2 thin films were deposited onto the ITO substrates. After annealing at 500ºC for 1 hour, the thin films were undergoing slow cooling at room temperature. 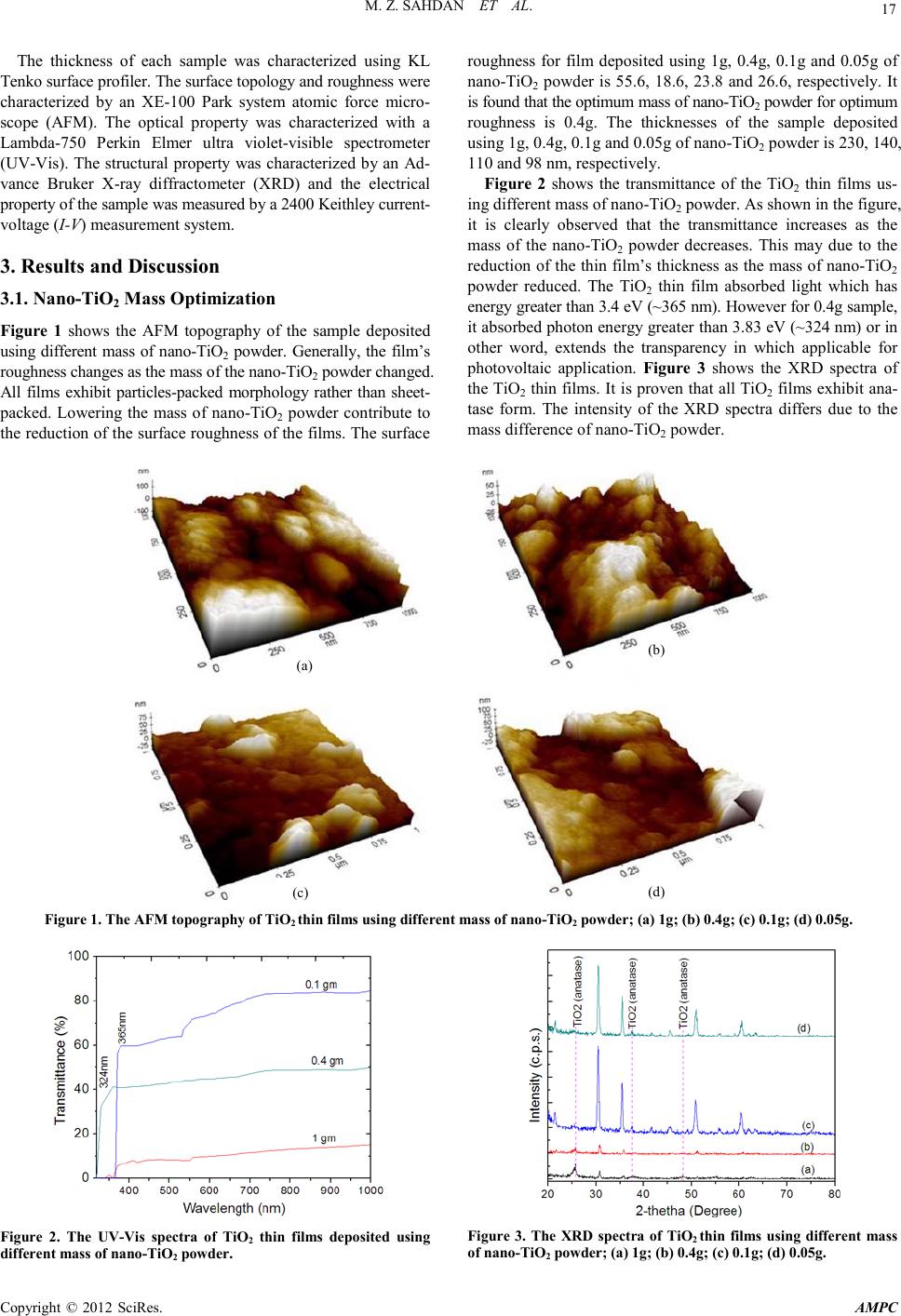 M. Z. SAHDAN ET AL. Copyright © 2012 SciRes. AMPC 17 The thickness of each sample was characterized using KL Tenko surface pro filer. The surface topology and roughness were characterized by an XE-100 Park system atomic force micro- scope (AFM). The optical property was characterized with a Lambda-750 Perkin Elmer ultra violet-visible spectrometer (UV-Vis) . The structural property was charact erized b y an Ad- vance Bruker X-ray diffractometer (XRD) and the electrical property of the sample was measured by a 2400 Keithley current - voltage (I-V) measurement system. 3. Results and Discussion 3.1. Nano-TiO2 Mass Optimization Figure 1 shows the AFM topography of the sample deposited using different mass of nano-TiO2 powder. Generally, the film’s roughness changes as the mass of the nano-TiO2 powder changed. All films exhibit particles-packed morphology rather than sheet- packed. Lo wering the mass o f nano-TiO2 powder contribute to the reduction of the surface roughness of the films. The surface roughness for film deposited using 1g, 0.4g, 0.1g and 0.05g of nano-TiO2 powder is 55.6, 18.6, 23.8 and 26.6, respectively. It is found that the optimum mass of nano-TiO2 powder for optimum roughness is 0.4g. The thicknesses of the sample deposited using 1g, 0.4g, 0.1g and 0.05g of nano-TiO2 powder is 230, 140, 110 and 98 nm, respectively. Figure 2 shows the transmittance of the TiO2 thin films us- ing different mass of nano-TiO 2 powder. As shown in the figure, it is clearly observed that the transmittance increases as the mass of the nano-TiO2 powder decreases. This may due to the reduction of the thin film’s thickness as th e mass of nano-TiO2 powder reduced. The TiO2 thin film absorbed light which has energy greater than 3.4 eV (~365 nm). However for 0.4g sample, it absorbed photon energy greater than 3.83 eV (~324 nm) or in other word, extends the transparency in which applicable for photovoltaic application. Figure 3 shows the XRD spectra of the TiO2 thin films. It is proven that all TiO2 films exhibit ana- tase form. The intensity of the XRD spectra differs due to the mass difference of n ano-Ti O2 powder. (a) (b) (c) (d) Figu re 1. The AFM topograph y of TiO2 thin films using different mass of nano-TiO2 powder; (a) 1g; (b) 0.4g; (c) 0.1g; (d) 0.05g. Figure 2. The UV-Vis spectra of TiO2 thin films deposited using different mass of nano-TiO2 powder. Figure 3. The XRD spectra of TiO2 thin films using different mass of nano-TiO2 powder; (a) 1g; (b) 0.4g; (c) 0.1g; (d) 0.05g.  M. Z. SAHDAN ET AL. Copyright © 2012 SciRes. AMPC 18 3.2. Acetic Acid Concentration Optimization Figure 4(a) shows the AFM topography of the sample deposited using 0.4g of nano-TiO2 powder without the presence of acetic acid catalyst. The surface roughness obtained from AFM is 32. Fig ure 4( b) shows the topography of TiO2 thin film when 3 ml of acetic acid was added in the solution. The surface roughness is reduced to 25.9. However, Figure 4(c) show different mor- phology of TiO2 thin film when 10 ml of acetic acid was used. The particle morphology is obviously seen and the surface roughness is reduced to 3.8 when the acetic acid volume was increased to 10 ml. On the other hand, Figure 4(d) shows al- most similar morphology with that of Figure 4(c). The surface roughness increased slightly to 4.9 when the acetic acid volume was 30 ml. It is found that the optimum acetic acid volume is 10 ml which results a uniform TiO2 thin films as shown in Figure 4(c). All samples exhibit almost similar thickness which is ap- proximately 130 nm. Figure 5 sh ows the trans mitt ance sp ectra of the s ample depo- sited using different acetic acid concen tration. Generall y, as the acetic acid volume increases, the transmittance of the TiO2 thin film also increased. However for 10 ml sample, the trans-mi ttance for wavelength from 419 to 547 nm decreased below the transmittance of 3 ml sample. The effect of adding acetic acid on the band gap i s evaluated u sing Tauc’s plot from the eq uations; ( ) [ ] 1ln 1tT α = × (1) and g E hc λ = (2) where α, t and T are the absorption coefficient, film’s thickness and transmittance, respectively. While Eg, h, c and λ are the energy gap, plank constant (4.136 × 10-15 eV), speed of light (3 × 108 m.s-1) and wavelength, respectively. It has been found that the band gap of the TiO2 thin films for 0, 3 and 30 ml sam- ples is around 3.2 eV. However, the 10 ml sample has different band gap value which is around 2.2 eV. Figure 6 shows the XRD sp ectra of the samples whi ch ind icates all TiO2 thin films are still in anatase form although the intensity is low. This low intensity of the film is due to the low thickness of TiO2 thin film. 3.3. Sensing Properties of TiO2 Thin Film In order to test current-voltage (I-V) char acteristic of the sample, Platinum (Pt) electrodes were deposited on the TiO2 thin film using a d.c. sputter coater. With Pt thickness around 15 nm, I-V probes were contacted and supplied with voltages from -2 V to +7 V using Keithley 2400 source meter. Figure 7 shows the I-V characteristic of the optimized TiO2 films (nano-TiO2 powder: 0.4g, acetic acid: 10 ml) when dropped with IPA. As shown in the figure, the TiO2 thin film exhibits Schottky response with Pt due to large difference of work function. The threshold voltage is around 6.7V. The threshold voltage in creased to 2 .4V as IP A was dropped on the thin film. The current value was gradually decreased by time and obviously seen after 30 second. The I-V response returned back to origin after 5 minutes. This phenomena is due to the chemical reaction between TiO2 particles and the IPA. The sensitivity of structural stability, porousity and surfa ce- to-volume ratio. TiO2 thin films prepared by sol-gel process provide a backbone that can be use as a microporous support in which analyte-sensitive species are trapped and into which analyte molecules may effecti vely dif fu s e and interact [11]. (a) (b) (c) (d) Figu re 4. The AFM topograph y of TiO2 thin films using diffe re nt acetic aci d conc entration; (a) 0 ml; (b) 3 ml; (c) 10 ml; (d) 30 ml.  M. Z. SAHDAN ET AL. Copyright © 2012 SciRes. AMPC 19 Figure 5. The transmittance spectra of TiO2 thin films using differ- ent acetic acid concentration; (a) 0 ml; (b) 3 ml; (c) 10 ml; (d) 30 ml . Figure 6. The XRD spectra of TiO2 thin films using diff erent acetic acid concentration; (a) 0 ml; (b) 3 ml; (c) 10 ml; (d) 30 ml. 4. Conclusion This paper presents the results of the optimization process to produce uniform and transparent TiO2 thin films using sol-gel technique. Two types of optimizations were performed. First was the mass of nano-TiO2 powder and second was the acetic acid co ncentr ation. The results from the AFM analysis confirmed that 0.4 g sample has the least TiO2 thin film roughness. Then by adding 10 ml of acetic acid has resulted optimum uniformity and roughness of the TiO2 thin film. The transmittance for the op- timum film is around 80% which is sufficient for optoelectronic application especially for solar cell. The XRD result indicates that all films are in anatase form. Finally, it has been demon- strated in this paper th at the prepared TiO2 thin film is sensitive towards o rganic so lvent whi ch could i ncrease th e current val ue. Therefore, it is applicab le for chemical s ensing appli cation. Figure 7. The sensin g property of TiO2 thin film toward IPA solvent. 5. Acknowledgements The authors would like to thank Universiti Tun Hussein Onn Malaysia for providing the technical supports and Ministry of Higher Education Malaysia (MOHE) for the financial support through fundamental research grant scheme (FRGS) vote No 1059 and MTUN COE res ear ch grant vote No C020. REFERENCES [1] S. Angkaew and P. Limsuwan, "Preparation of silver-titanium dioxide core-shell (Ag@TiO2) nanoparticles: Effect of Ti-Ag mole ratio," Proce di a Engine er i ng, vol. 32, pp. 649-655 , 2012. [2] V. Brezová, et al., "Photoactivity of mechanochemically pre- pared nanoparticulate titanium dioxide investigated by EPR spectroscopy," Journal of Photochemistry and Photobiology A: Chemistry, vol. 206, pp. 177-187, 2009. [3] R. K. Keswani, et al., "Room temperature synthesis of titanium dioxide nanoparticles of different phases in water in oil micro- emulsion," Colloids and Surfaces A: Physicochemical and Engi- ne ering Aspects , vol. 369 , pp. 75-81, 2010. [4] A. Kiselev, et al., "Solar light decomposition of DFP on the surface of anatase and rutile TiO2 prepared by hydrothermal treatment of microemulsions," Surface Science, vol. 584, pp. 98-105, 2005. [5] J. Taranto, et al., "Photocatalytic air purification: Comparative efficac y and pressu re drop of a TiO2-c oated thin mesh and a ho- neycomb monolith at high air velocities using a 0.4 m3 close-loop rea ctor," Separation and Purification Technology, vol. 67, pp. 187-193, 2009. [6] J. Moon, et al., "Pd-doped TiO2 nanofiber networks for gas sensor applications," Sensors and Actuators B: Chemical, vol. 149, pp. 301-305, 2010. [7] A. L. Ahmad, et al., "Synthesis and characterization of TiO2 membrane with palladium impregnation for hydrogen separa- tion," Journal of Membrane Science, vol. 366, pp. 166-175, 2011. [8] S. Nad, et al., "Anomalous nanostructured titanium dioxide," Journal of Colloid and Interface Science, vol. 264, pp. 89-94, 2003. [9] R . Gup t a, et al., "E ffect of et hanol vari ation on the int ernal en vi- ronment of sol–gel bulk and thin films with aging," Biosensors  M. Z. SAHDAN ET AL. Copyright © 2012 SciRes. AMPC 20 and Bi o e le ctron ic s, vol. 21, pp. 549-556, 2005. [10] J. Calabria A, et al., "Synthesis of sol–gel titania bactericide coatings on adobe brick," Construction and Building Materials, vol. 24, pp. 384-389, 2010. [11] S. H. Si, et al., "Improvement of piezoelectric crystal sensor for the detection of organic vapors using nanocrystalline TiO2 films," Sensors and Actuators B: Chemical, vol. 108, pp. 165-171, 2005. |

