Paper Menu >>
Journal Menu >>
 Open Journal of Blood Diseases, 2012, 2, 95-99 http://dx.doi.org/10.4236/ojbd.2012.24018 Published Online December 2012 (http://www.SciRP.org/journal/ojbd) 95 CD4+/CD56+ Hematodermic Neoplasm Presenting in the Skin: A Tunisian Case Report and Current Review of the Literature Yosra Ben Youssef1, Nessrine Ben Sayed1, Kmira Zahra1, Abir Gmidène2, Naouel Ben Salah3, Atef Ben Abdelkader4, Nejia Brahem5, Hlima Sennana2, Colanda Belajouza6, Abderrahim Khelif1 1Department of Clinical Hematology, Farhat Hached University Hospital, Sousse, Tunisia; 2Department of Cytogenetics, Farhat Hached University Hospital, Sousse, Tunisia; 3Department of Cytology, Aziza Othmana University Hospital, Tunis, Tunisia; 4De- partment of Anatomopathology, Farhat Hached University Hospital, Sousse, Tunisia; 5Department of Cytology, Farhat Hached Uni- versity Hospital, Sousse, Tunisia; 6Department of Dermatology, Farhat Hached University Hospital, Sousse, Tunisia. Email: yosra.benyoussef@laposte.net, nessrinebensayed@yahoo.fr, kmirazahra@yahoo.fr, gmidene_abir@yahoo.fr, abkheli@gmail.com Received October 10th, 2012; revised November 15th, 2012; accepted November 25th, 2012 ABSTRACT The CD4+/CD56+ hematodermic neoplasm is a rare aggressive systemic neoplasm for which effective therapies have not yet been established, it is clinically characterized by cutaneous involvement with spread to bone marrow, blood and poor prognosis with current chemotherapy regimens. Our objective is to report diagnosis and treatment difficulties of CD4+/CD56+ hematodermic neoplasm. We describe here a Tunisian man who presented with subcutaneous ulcerated lesion localized in the right leg and multiples generalized nodules. Skin biopsy showed an atypical lymphoid cell infil- tration with an angiocentric pattern and extensive necrosis by immuno-histochemical analysis, these cells were positive for CD4, CD56, granzyme B and negative for CD8, CD123, CD20 and CD30. T-cell rearrangement and Ep- stein-Barr-virus (EBV) in situ hybridation studies were negative. The patient underwent 5 cycles chemotherapy SMILE regimen monthly sandwiched with radiotherapy on the residual lesions of the right leg with great tolerance but he re- lapsed within 8months with skin, blood, bone marrow, lung, and cerebrospinal involvement. Based on these findings, the patient was diagnosed with CD4+/CD56+ hematodermic neoplasm (blastic NK-like T-cell lymphoma) treated with one course of hyper-CVAD regimen, he died within 20 days with a septic chok. Despite the use of L-Asparaginase and radiotherapy the prognosis is very poor; we suggest the exploration for highly active drugs, hematopoietic stem cell transplantation (HSCT) is crucial to improve survival. Keywords: Blastic NK-Like T-cell Lymphoma; Hyper-CVAD; SMILE; Prognosis; Treatment 1. Introduction Since the early 1990s, several cases of CD4+ CD56+ hematodermic neoplasm’s (HN) (WHO-EORTC) have been reported. Based on the lymphoid morphology of tumor cells, expression of CD56 (also known as neural cell adhesion molecule NCAM) and the absence of the T-cell receptor or surface CD3, a natural killer (NK) ori- gin has been suggested, despite the negativity of most NK-associated markers and the absence of azurophilic granulations. These tumors fell under the designation of blastic NK-like T-cell lymphoma (WHO) without an evidence of a NK-cell origin [1]. From 1993 to 2011, 64 cases of cutaneous lymphoma were diagnosed in our hospital, with only one case of blastic NK-like T-cell lymphoma of the skin in 2011. Due to the rarity of this entity, we report a case of a 49-year old man with blastic NK-like cell lymphoma with skin, bone marrow, blood, cerebrospinal fluid involvement and reviewed clinical manifestations, treatment and prognosis of this condition. 2. Case Report A 49-year-old Tunisian man developed slowly growing subcutaneous 15 cm nodules secondly ulcerated localized in the right leg. Subsequently similar nodules appeared on the trunk, back, the other leg and upper extremities. At the time of evaluation, about 10 red nodules from in- dex finger seized to thumb seized and homolateral in- guinal lymphadenopathy were noted (Figures 1(A)-(C)). Skin biopsy showed atypical lymphoid cell infiltration with an angiocentric pattern and extencive necrosis. These cells were positive for CD4, CD56, CD123 granzyme B, and negative for CD8, terminal deoxynucleotidyl trans- Copyright © 2012 SciRes. OJBD 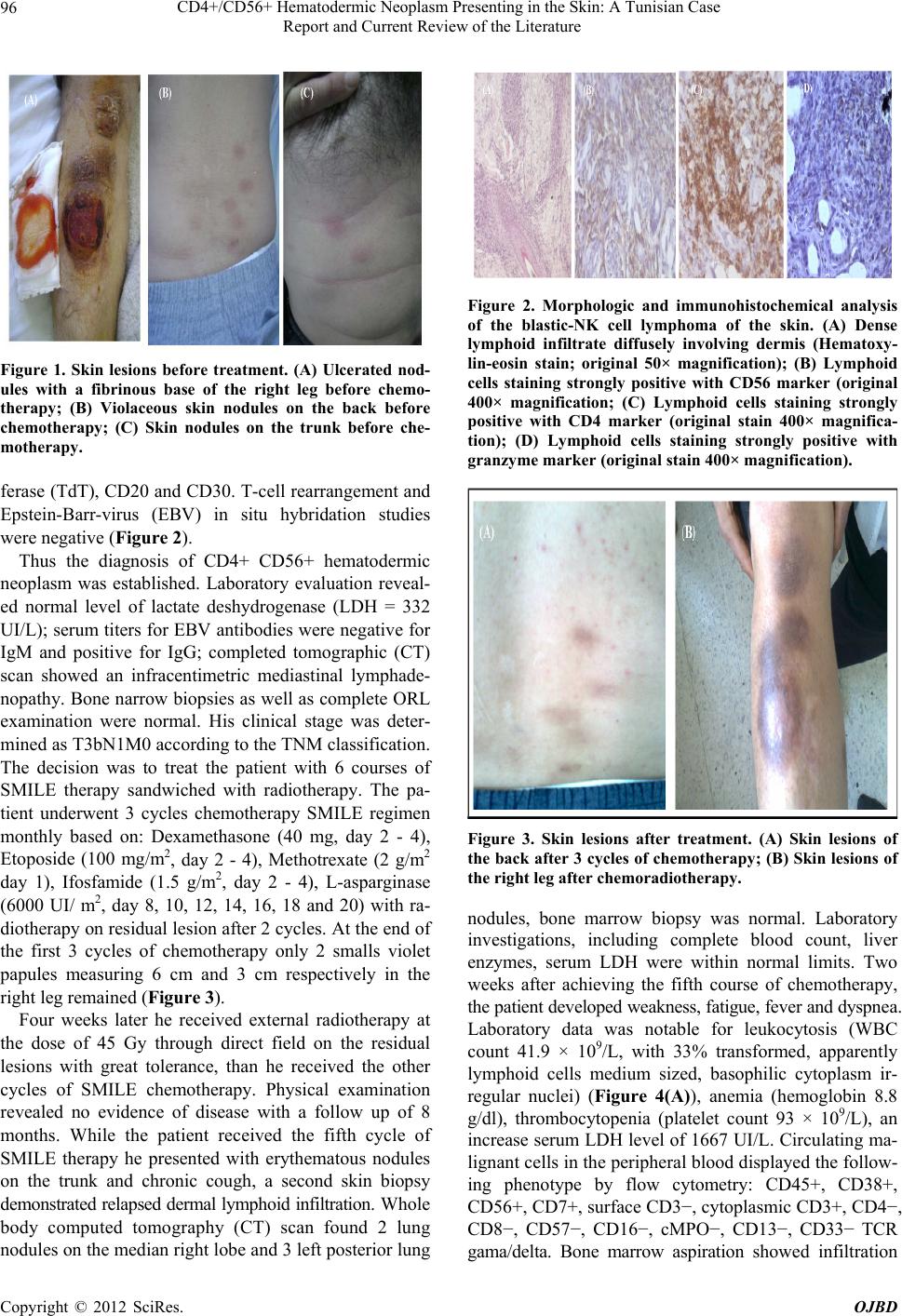 CD4+/CD56+ Hematodermic Neoplasm Presenting in the Skin: A Tunisian Case Report and Current Review of the Literature 96 Figure 1. Skin lesions before treatment. (A) Ulcerated nod- ules with a fibrinous base of the right leg before chemo- therapy; (B) Violaceous skin nodules on the back before chemotherapy; (C) Skin nodules on the trunk before che- motherapy. ferase (TdT), CD20 and CD30. T-cell rearrangement and Epstein-Barr-virus (EBV) in situ hybridation studies were negative (Figure 2). Thus the diagnosis of CD4+ CD56+ hematodermic neoplasm was established. Laboratory evaluation reveal- ed normal level of lactate deshydrogenase (LDH = 332 UI/L); serum titers for EBV antibodies were negative for IgM and positive for IgG; completed tomographic (CT) scan showed an infracentimetric mediastinal lymphade- nopathy. Bone narrow biopsies as well as complete ORL examination were normal. His clinical stage was deter- mined as T3bN1M0 according to the TNM classification. The decision was to treat the patient with 6 courses of SMILE therapy sandwiched with radiotherapy. The pa- tient underwent 3 cycles chemotherapy SMILE regimen monthly based on: Dexamethasone (40 mg, day 2 - 4), Etoposide (100 mg/m2, day 2 - 4), Methotrexate (2 g/m2 day 1), Ifosfamide (1.5 g/m2, day 2 - 4), L-asparginase (6000 UI/ m2, day 8, 10, 12, 14, 16, 18 and 20) with ra- diotherapy on residual lesion after 2 cycles. At the end of the first 3 cycles of chemotherapy only 2 smalls violet papules measuring 6 cm and 3 cm respectively in the right leg remained (Figure 3). Four weeks later he received external radiotherapy at the dose of 45 Gy through direct field on the residual lesions with great tolerance, than he received the other cycles of SMILE chemotherapy. Physical examination revealed no evidence of disease with a follow up of 8 months. While the patient received the fifth cycle of SMILE therapy he presented with erythematous nodules on the trunk and chronic cough, a second skin biopsy demonstrated relapsed dermal lymphoid infiltration. Whole body computed tomography (CT) scan found 2 lung nodules on the median right lobe and 3 left posterior lung Figure 2. Morphologic and immunohistochemical analysis of the blastic-NK cell lymphoma of the skin. (A) Dense lymphoid infiltrate diffusely involving dermis (Hematoxy- lin-eosin stain; original 50× magnification); (B) Lymphoid cells staining strongly positive with CD56 marker (original 400× magnification; (C) Lymphoid cells staining strongly positive with CD4 marker (original stain 400× magnifica- tion); (D) Lymphoid cells staining strongly positive with granzyme marker (original stain 400× magnification). Figure 3. Skin lesions after treatment. (A) Skin lesions of the back after 3 cycles of chemotherapy; (B) Skin lesions of the right leg after chemoradiotherapy. nodules, bone marrow biopsy was normal. Laboratory investigations, including complete blood count, liver enzymes, serum LDH were within normal limits. Two weeks after achieving the fifth course of chemotherapy, the patient developed weakness, fatigue, fever and dyspnea. Laboratory data was notable for leukocytosis (WBC count 41.9 × 109/L, with 33% transformed, apparently lymphoid cells medium sized, basophilic cytoplasm ir- regular nuclei) (Figure 4(A)), anemia (hemoglobin 8.8 g/dl), thrombocytopenia (platelet count 93 × 109/L), an increase serum LDH level of 1667 UI/L. Circulating ma- lignant cells in the peripheral blood displayed the follow- ing phenotype by flow cytometry: CD45+, CD38+, CD56+, CD7+, surface CD3−, cytoplasmic CD3+, CD4−, CD8−, CD57−, CD16−, cMPO−, CD13−, CD33− TCR gama/delta. Bone marrow aspiration showed infiltration Copyright © 2012 SciRes. OJBD  CD4+/CD56+ Hematodermic Neoplasm Presenting in the Skin: A Tunisian Case Report and Current Review of the Literature 97 with 10% centroblastoid cells with irregular nuclei and inconspicuous nucleoli (Figure 4(B)). Bone marrow bi- opsy showed massive infiltration with medium-sized blasts (Figure 4(C)). Examination of cerebrospinal fluid showed infiltration with lymphoid blastic cells. Cytogenetic analysis re- vealed complex karyotype with 46, XY, del (11)(p14), del (12)(p13) [4]/45, XY, add (1)(p36), del (12)(p13), −22 [3]/45, XY, add (2)(p24), del (12)(p13), −15 [2]/46, XY [11]. Based on these findings, the patient received the first course of hyper-CVAD regimen (fractionated cyclopho-sphamide, vincristine, doxorubicin, and dexa- methasone), with central nervous system prophylaxis using intrathecal methotrexate/cytarabine, he died within 20 days with a septic chok. 3. Discussion Natural killer (NK) and NK-like T-cell lymphoma are aggressive hematologic malignancies that have an ex- tranodal presentation. The main affected organ sites in- clude the gastrointestinal tract, skin and nasal cavities. These neoplasms are categorized into nasal versus non nasal type; they can present initially in the skin and/or involve the skin as a part of a multiorgan disseminated lymphoma. In both NK and NK-like T-cell lymphomas, the neoplastic cells express CD2 and CD56. The distinc- tion of NK versus NK-like T-cell lymphoma is based on the T-cell receptor (TCR) β and/or γ gene rearrangement and surface CD3 expression; those that lack these fea- tures are categorized by NK lymphomas, those that manifest surface CD3 and exhibit a TCR rearrangement are considered NK-like T-cell lymphomas. The expre- ssion of CD4 is designed as blastic NK-like T-cell lym- phoma. More recently, it has been established that the Figure 4. Morphologic analysis of the blastic NK-cell lym- phoma in the blood and bone marrow; (A) Lymphoid me- dium-sized cells, basophilic cytoplasm, irregular nuclei (Blood) (May-Grunwald-Giemsa staining); (B) Bone mar- row aspiration: centroblastoid cells with irregulae nuclei, in-conspicuous nucleoi (May-Grunwald-Giemsa staining); (C) Medium-sized blasts infiltrating the bone marrow bi- opsy (hematoxylin eosin, ×100). cell of origin is a plasmacytoid/dendritic cells, cones- quently the term “blastic NK-like T-cell lymphomas has been supplanted by the term “CD4+ CD56+ hematoder- mic neoplasm” in the WHO/EORTC classification but is now termed blastic plasmacytoid dendritic cell neo- plasm(BPDCN) [2,3]. Blastic NK-like T-cell lymphoma can arise at any age including childhood, but it tends to present in middle aged or elderly patients with a median age of 52 years [3-5]. It affects predominantly man than woman with a sex ratio 3:1 [5], there is no racial predi- lection [6]. Similar to acute leukemia, blastic NK-cell leukemia/lymphoma has a multiorgan involvement, lym- phadenopathy, splenomegaly with a rapid blood and bone marrow involvement [7]. Cutaneous lesions may be solitary or multiple nodules or tumors, or have petechial appearance witch tend to be generalized [3,7-9]. Skin biopsy reveals a dense dermal infiltration by medium malignant cells without epidermal involvement with fo- cal angiocentric accentuation and necrosis [2,4,7]. Im- munophenotypically, blastic NK-cell lymphoma is typi- cally CD45+, CD2+, surface CD3−, cytoplasmic CD3+, CD4+, CD56+, CD5−, CD7+/−, TdT+/−TCR genes are in germline configuration [2,7]. The absence of positivity for myeloperoxidase, CD13 and CD33 in bone marrow cells excluded the diagnosis of CD56+ myeloid leukemia [4,10]. In contrast with aggressive NK-cell leukemia and extranodal NK/T-cell lymphomas, EBV DNA in tumor cells is negative, so the possibility of extranodal NK/ T-cell lymphoma nasal type is excluded and the positiv- ity of EBV might serve as a key point for differential diagnosis from mature NK cell malignancies [8,9]. Cy- togenetic analysis reveals a complex karyotype, such as deletion of chromosomes 9, 13, 15, deletion of the 5q, 12p and 6q abnormalities, but no recurring abnormalities were identified [7]. Deletion of 12p and add 1 were found in our patient. Suzuki et al. reported 4 cases of blastic NK cell lymphoma, only one patient had cytoge- netic abnormalities [10]. Differential diagnosis includes other CD56+ lymphomas with cutaneous involvement. These include extranodal NK/T-cell lymphoma (ENKTL) nasal type, CD56+ myeloid leukemia, cutaneous CD30+ lymphoproliferation with CD56 expression and cutane- ous granulocytic sarcoma [2,8]. The prognosis of patients with blastic NK-like T-cell lymphoma is poor with a me- dian survival of 14 months, 2 and 5 years overall survival of 33% and 6% respectively [7,8]. There is no standard treatment for this malignancy, response to combination chemotherapy such as CHOP, is minimal or transient and early progression is common. Radiotherapy is effective for localized disease only [8]. More aggressive therapies directed to allogenic stem cell transplantation are proba- bly superior with long term remission [8]. Several studies showed efficacy of regimens containing L-Asparaginase. Copyright © 2012 SciRes. OJBD  CD4+/CD56+ Hematodermic Neoplasm Presenting in the Skin: A Tunisian Case Report and Current Review of the Literature 98 The rationale is based on reports that L-Asparaginase is effective in the treatment of NK/T-cell lymphoma [8,11]. L-Asparaginase induced selective apoptosis of NK-lym- phoma cells in vitro, these results in fast inhibition of DNA and RNA synthesize in lymphocytes [5]. Etoposide has demonstrated in vitro and in vivo efficacy. Increased expression of a multidrug resistant (MDR) phenotype has been correlated with aggressive behavior in these lym- phomas [3,5,6,10]. MDR phenotype and P53 mutation also can be associated with a poor prognosis and may help predict response to the treatment. [3,5,6,10]. The components of SMILE protocol are MDR unrelated agents and EBV associated lymphoproliferative disorders. Motoko et al. reported an overall response rate at 67% for six Japanese patients with advanced-stage, relapsed or refractory ENKT-CL and leukemia treated with six courses of chemotherapy SMILE regimen with sand- wiched radiotherapy after three courses [12]. Hyper- CVAD regimen is used mainly for therapy of acute lym- phocyte leukemia and high-grade lymphomas. Shapiro et al. reported successfully achieved complete remissions in two patients using the hyper-CVAD regimen [7]. Among five patients with CD4+/CD56+ HN, one patient treated with the hyper-CVAD regimen achieved a complete re- sponse and remain alive with a follow-up of over than 38 months [13]. Allogenic HSCT, with the potential benefit of graft-versus lymphoma effect is a second option for patients with advanced disease, long remission of blastic NK-cell lymphoma was reported after autologous pe- ripheral blood stem cell transplantation. Therefore al- logenic or autologous, or cord blood stem cell transplant- tation is a promising treatment strategy, but small series have shown that it is a potentially curative option [7,8, 14,15]. Age ≤40 years, presentation with only skin le- sions, initial treatment with transplantation directed regimens and TdT expression by >50% of the neoplastic cells were associated with better prognosis [2]. 4. Conclusion CD56+ CD4+ HN’s are an extremely difficult group, for pathologists and clinicians. For pathologists, correct clas- sification of these lymphomas is difficult, expensive and time-consuming, it requires application of several com- plimentary techniques such as extensive phenotyping, EBV analysis and T-cell receptor TCR gene rearrange- ment studies. Clinicians are confronted with an aggres- sive clinical behavior and a fatal outcome often within 1 year after diagnosis, and may consider more intensive therapies, as in acute leukemias, as initial therapy. Pro- spective data on larger series of patients treated homo- geneously also with innovative approaches are needed in order to establish the best treatment for this disease. REFERENCES [1] R. Dummer, K. Asagoe, A. Cozzio, G. Burg, U. Doeb- beling and P. Golling, “Recent Advances in Cutaneous Lymphomas,” Journal of Dermatological Science, Vol. 48, No. 3, 2007, pp. 157-167. doi:10.1016/j.jdermsci.2007.09.001 [2] C. M. Magro, P. Porcu, J. S. Schaefer, J. W. Erter, R. R. Furman, P. K. Shitabata, et al., “Cutaneous CD4+ CD56+ Hematologic Malignancies,” Journal of the American Academy Dermatology, Vol. 63, No. 2, 2010, pp. 292-308. doi:10.1016/j.jaad.2009.08.044 [3] R. G. Asher and K. Hollowood, “Primary Cutaneous Lymphoma: An Overview Based on the WHO-EORTC Classification, Mini-Symposium: Haematopathology Up- date III,” Diagnostic Histopathology, Vol. 16, No. 4, 2010, pp. 168-181. [4] K. K. Sra, J. W. Labiche, R. Rapini, R. Jordon, S. Raimer and S. Tyring, “T/Natural Killer-Cell Lymphomas,” Jour- nal of the American Academy Dermatology Letters, 2005, pp. 708-709. [5] M. W. Bekkenk, P. M. Jansen, C. J. L. M. Meijer and R. Willemze, “CD56+ Hematological Neoplasms Presenting in the Skin: A Retrospective Analysis of 23 New Cases and 130 Cases from the Literature,” Annals of Oncology, Vol. 15, No. 7, 2004, pp. 1097-1108. doi:10.1093/annonc/mdh268 [6] Y. L. Kwong, “Natural Killer-Cell Malignancies: Diag- nosis and Treatment,” Leukemia, Vol. 19, No. 12, 2005, pp. 2186-2194. doi:10.1038/sj.leu.2403955 [7] M. Shapiro, M. A. Wasik, J. M. Junkins-Hopkins, A. H. Rook, C. C. Vittorio, H. Ikatura, et al., “Complete Re- mission in Advanced Blastic NK-Cell Lymphoma/Leu- kemia in Elderly Patients Using the Hyper-CVAD Regi- men,” American Journal of Hematology, Vol. 74, No. 1, 2003, pp. 46-51. doi:10.1002/ajh.10381 [8] J. W. Tjiu and C. H. Hsiao, “Blastic Natural Killer-Cell Lymphoma Presenting in the Skin,” Tzu Chi Medical Journal, Vol. 19, No. 3, 2007, pp. 173-178. http://health.elsevier.com/tcmj [9] R. P. Falcao, A. B. Garcia, M. G. Marques, B. P. Simoes, B. A. Fonseca and M. L. Rodrigues, et al., “Blastic CD4 NK Cell Leukemia/Lymphoma: A Distinct Clinical En- tity,” Leukemia Research, Vol. 26, No. 9, 2002, pp. 803-807. [10] R. Suzuki and S. Nakamura, “Malignancies of Natural Killer (NK) Cell Precursor: Myeloid/NK Cell Precursor Acute Leukemia and Blastic NK Cell Lymphoma/ Leu- kemia,” Leukemia Research, Vol. 23, No. 7, 1999, pp. 615-624. [11] J. V. E. Reyes, T. Al-Saleem, V. G. Robu and M. R. Smith, “Extranodal NK/T-cell Lymphoma Nasal Type: Efficacy of Pegaspargase. Report of Two Patients from the United States and Review of Literature,” Leukemia Research, Vol. 34, No. 1, 2010, pp. 50-54. doi:10.1016/j.leukres.2009.09.002 [12] M. Yamaguchi, R. Suzuki, Y. L. Kwong, W. S. Kim, Y. Hasegawa, J. I. Kiya, et al., “Phase I Study of Dexa- methasone, Methotrexate, Ifosfamide, L-Asparaginase Copyright © 2012 SciRes. OJBD  CD4+/CD56+ Hematodermic Neoplasm Presenting in the Skin: A Tunisian Case Report and Current Review of the Literature Copyright © 2012 SciRes. OJBD 99 and Etoposide (SMILE) Chemotherapy for Advanced- Stage, Relapsed or Refractory Extranodal Natural Killer (NK)/T-cell Lymphoma and Leukemia,” Cancer Science, Vol. 99, No. 5, 2008, pp. 1016-1020. doi:10.1111/j.1349-7006.2008.00768.x [13] P. Ng Ashley, L. Stephen, R. Timothy, Mc. Cormack, C. Prince and H. M. D. Awesterman, “Primary Cutaneous CD4+/CD56+ Hematodermic Neoplasm (Blastic NK-Cell Lymphoma): A Report of Five Cases,” Hematologica, Vol. 91, No. 1, 2008, pp. 143-144. http://www.haematologica.org/journal/2006/01/143html [14] J. J. Leitenberger, C. N. Berthelot, K. D. Polder, P. McLaughlin, D. Jones and M. Duvic, “CD4+ CD56+ Hematodermic/Plasmacytoid Dendritic Cell Tumor with Response to Pralatrexate,” Journal of the American Aca- demy Dermatology, Vol. 58, No. 3, 2008, pp. 480-484. doi:10.1016./jaad.2004 [15] E. C. Parlette, Z. Elliott, F. W. Hall and B. S. Grabam, “Primary Cutaneous Blastic Natural Killer Cell Lym- phoma,” Journal of the American Academy Dermatology, Vol. 53, No. 4, 2005, pp. 742-743. |

