Materials Sciences and Applications
Vol.4 No.7(2013), Article ID:33804,13 pages DOI:10.4236/msa.2013.47051
Low Temperature Chlorination of Nd2O3 by Mechanochemical Method with CCl4
![]()
1Nuclear Fuel Cycle Engineering Laboratories, Japan Atomic Energy Agency, Naka, Japan; 2Institute of Multidisciplinary Research for Advanced Materials, Tohoku University, Sendai, Japan.
Email: nagai.takayuki00@jaea.go.jp
Copyright © 2013 Takayuki Nagai et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received April 8th, 2013; revised May 19th, 2013; accepted May 30th, 2013
Keywords: Mechanochemical Method; Carbon Tetrachloride; Neodymium Oxide; Low Temperature Chlorination; Neodymium Oxychloride; XRD; RAMAN Spectroscopy
ABSTRACT
For a chlorinating method at low temperature, the possibility of chlorination of Nd2O3 by a mechanochemical reaction with CCl4 was studied using a planetary ball mill. The mechanochemical experiments were conducted by changing the pot materials, milling time, molar ratio of CCl4/Nd2O3, and revolution speed. As the results of obtained products by X-ray diffractometry and Raman spectroscopy, it was confirmed that the chlorination to NdOCl from Nd2O3 with CCl4 was advanced at room temperature in a zirconia or tungsten pot with balls. We found that an extension of the milling time and an increase of the number of ball were effective to the chlorination to NdOCl and that tensile stress remained in the milled powder by using a planetary ball mill.
1. Introduction
In the non-aqueous reprocessing process for spent nuclear metallic fuels by the pyro-metallurgical method [1], a spent fuel is dissolved into the LiCl-KCl eutectic melt at 450˚C - 500˚C and dissolved uranium and plutonium are collected as metals by an electrolysis technique. By the contamination of oxide impurities into the process environment, these actinide ions are de-chlorinated and precipitated in the melt as oxides. In order to decrease this precipitation of actinide oxides, the atmosphere in process devices is needed to be filled with inert gas in high purity, but the contamination of oxide impurities to the atmosphere cannot prevent completely. Therefore, the study on the dissolution of actinide oxides into the melt has been conducted as the chlorination process development. In the current study [2], the uranium oxides in the LiCl-KCl eutectic melt were chlorinated to uranium chlorides by the addition of zirconium tetra-chloride, ZrCl4, but zirconia particles were generated by this chlorination. When this adding ZrCl4 method is assumed to apply to the practical process, it is necessary to establish the efficient technique which can separate the zirconia particles from the melt at high temperature. This development is not easy though the centrifuge is promising as this separation technique.
It is thought that the chlorinating operation of actinide oxides is appropriate at the refining treatment process of used solvent salts. This refining process is a batch operation, and the assumed procedure is as follows: After settling the melt in the electrolytic vessel, the top clear melt is transported to the other vessel, and then the rest of melt including actinide oxides is coagulated. This coagulated salt is taken out, and the actinide oxides with coexistence of solvent salt are chlorinated by the new chlorination method.
For this chlorination method, we are examining the possibility of using a mechanochemical reaction in easier operating conditions at low temperature. In the present study, the possibility of low temperature chlorination of di-neodymium tri-oxide, Nd2O3, with carbon tetrachloride, CCl4, was studied by using a planetary ball mill. For this mechanochemical method, the sulfurization of Nd2O3 with carbon disulfide, CS2, to neodymium oxysulfide, Nd2O2S, had been confirmed at the low temperature [3]. Many studies by using a planetary ball mill were carried out, and those results were reported about the mechanical alloying [4-7], the physical effect investigation [8-10], the mechanochemical synthesis [3,11-15], etc. However, no study has been reported on the low temperature chlorination of rare-earth oxides by mechanochemical treatment using CCl4. This method would be a potential process for the low temperature synthesis of metal oxide, especially lanthanides and actinides. In this text, the mechanochemical experiments of Nd2O3 with CCl4 and the X-ray diffraction (XRD) patterns and Raman spectra of their mechanochemical products are described.
2. Experimental
2.1. Reagents
Anhydrous oxide powder of Nd2O3 with 99.9% in purity was purchased from Kojundo Chemical Lab. Co., Ltd., and it was treated by vacuum drying for 2 h at 200˚C before the use. A reagent CCl4 with a boiling point of 76˚C - 77˚C and maximum water content of 0.02% was purchased from Wako Pure Chemical Industries, Ltd. and was used without further treatment.
2.2. Apparatus
A planetary micro mill “Pulversettle 7” of Fritsch GmbH, which equipped with a pair of two pots and balls, was used for these mechanochemical experiments. Three kinds of pair pots which were made from SUS304 stainless steel, zirconia, or tungsten carbide were used. All pots have a vessel space with 40 mm in height and 40 mm in diameter, as shown in Figure 1. The used balls were made from the same material of pots. The densities of SUS304, zirconia, and tungsten carbide are 7.9, 6.0, and 15 g∙cm−3, respectively.
2.3. Experimental Procedure
The Nd2O3 powder, CCl4 liquid and balls were put in one of pair pots, and only the balls were put in another pot. The added amounts of Nd2O3 and CCl4 in the pot are adjusted to molar ratio of CCl4/Nd2O3 with 3.47 in the
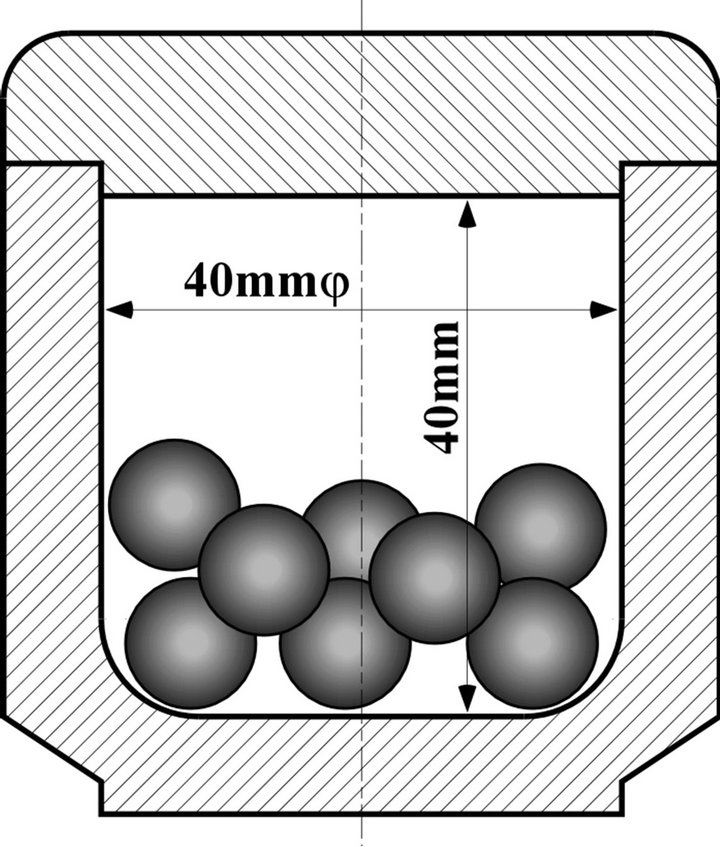
Figure 1. Schematic cross section of pot and balls in planetary ball mill.
standard condition of this study. After setting a pair pots in the planetary ball mill, the mill was operated for 0.5 - 4 h at room temperature with the revolution speed of planetary disk at 250 or 500 rpm. The transmission ratio between the revolution speed of planetary disk and the rotation speed of milling pots was 1:−2. And the rotation of the pots was set as the opposite direction to the revolution of the planetary disk, as illustrated in reference [9]. The milling operation interrupted for 15 minutes every 30 minutes milling to avoid overheating by a mechanochemical reaction. The mechanochemical experiments for Nd2O3 with CCl4 were carried out in 19 conditions as shown in Table 1. The number of balls in a pot was selected in consideration of the standard condition described in the catalog of the planetary ball mill. However, in using a tungsten carbide pot, the number of ball was a half of the standard condition to suppress the load to the planetary ball mill.
After the milling operation, the XRD pattern of products, which were obtained by mechanochemical experiments, was measured in a continuous scan mode between 10˚ and 60˚ in 2θ by using the X-ray powder diffractometer Type RAD-IC of Rigaku Co. with a CuKα irradiation source at 40 kV and 20 mA monochromatized by pyrolytic graphite. Raman spectra of the products were obtained using the laser Raman spectrometer NRS-5100 of JASCO Co. and were measured in wavenumber range of 100 - 600 cm−1 for 5 s by excitation of 532 nm laser beam with 6.4 - 7.4 mW.
3. Results and Discussions
3.1. Products Obtained from Nd2O3 with CCl4 by Using a Planetary Ball Mill
Figure 2 shows the XRD patterns of products obtained by a milling of Nd2O3 reagent powder with CCl4 for 1 h at room temperature. The XRD pattern of Nd2O3 powder before a milling showed sharp peaks as shown in Figure 2(a). The positions of their peaks at 26.8˚, 29.8˚, 30.8˚, 40.5, 47.4˚, 53.5˚, 56.9˚, and 57.6˚ were corresponding to the International Centre for Diffraction Data JCPDS41- 1089 of Nd2O3, and the other small peaks at 15.9˚, 27.8˚, 28.7˚, and 49.7˚ were identified as neodymium tri-hydroxide, Nd(OH)3, of JCPDS83-2035. The reason that Nd(OH)3 was identified in the Nd2O3 reagent, was thought that Nd2O3 was prepared by a wet method. Bernal et al. had observed that the XRD pattern of Nd(OH)3 in the Nd2O3 powder increased with an increase of the exposure time in air [16]. When the Raman spectrum of the Nd2O3 reagent was measured as shown in Figure 3(a), the vibration bands by the Nd2O3 trigonal symmetry crystal observed at 106, 428, and 436 cm−1 [17]. Moreover, the color of Nd2O3 is light blue and Nd(OH)3 shows a light violet. The Nd2O3 reagent showed a light blue and
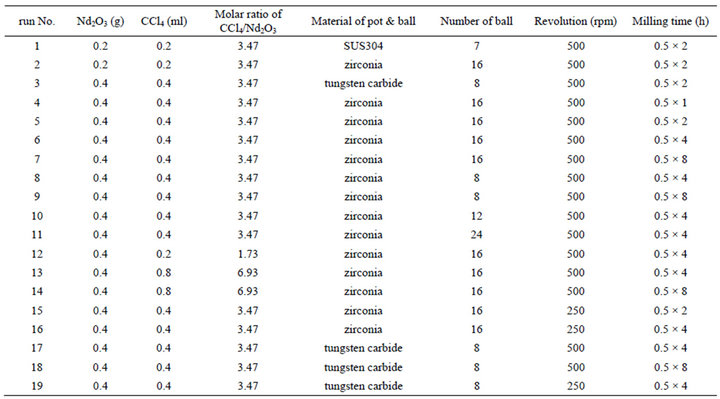
Table 1. Experiment condition by mechanochemical reaction.
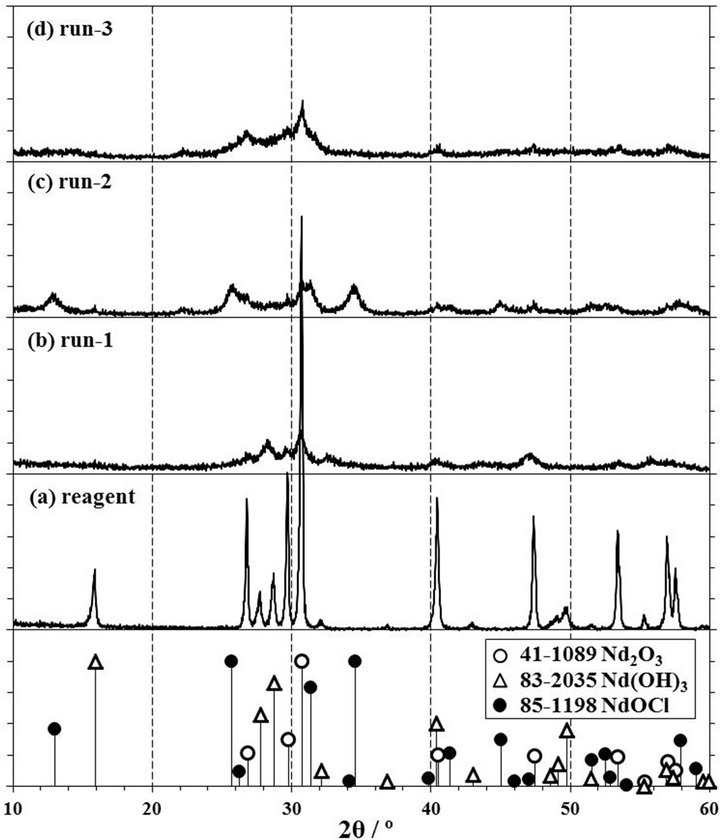
Figure 2. XRD patterns of (a) Nd2O3 reagent and (b)-(d) products by milling for 1 h at 500 rpm. The product of (b) run-1 was operated in SUS304 pot with 15 mmφ × 7 balls, that of (c) run-2 was in zirconia pot with 10 mmφ × 16 balls, and that of (d) run-3 was in tungsten carbide pot with 10 mmφ × 8 balls. 1 span of Y-axis is 100 cps.
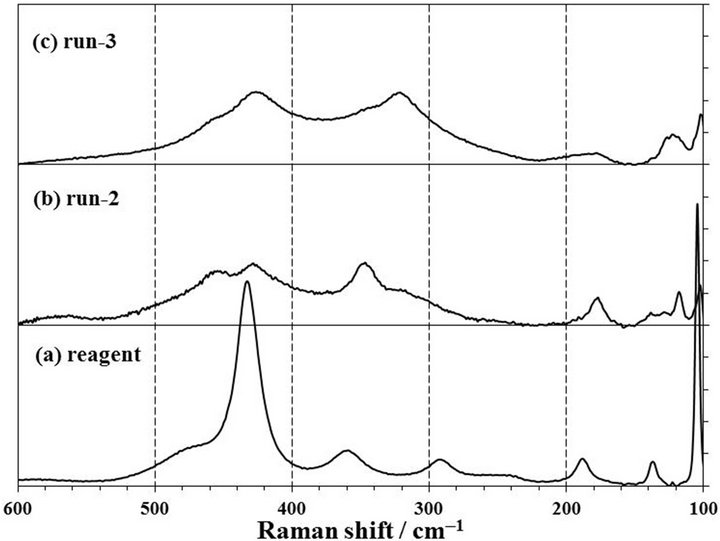
Figure 3. Raman spectra of (a) Nd2O3 reagent, (b) and (c) products by milling for 1 h at 500 rpm. The product of (b) run-2 was in zirconia pot with 10 mmφ × 16 balls and that of (c) run-3 was in tungsten carbide pot with 10 mmφ × 8 balls. 1 span of Y-axis is 25 counts.
Nd(OH)3 was thought not to be contained so much in the Nd2O3 reagent.
As for the product by a milling at 500 rpm for 1 h in a SUS304 pot with 15 mmφ × 7 balls of run-1, the product discolored to blackish from light blue of Nd2O3. The XRD pattern of this product was extremely gradual as shown in Figure 2(b), and new broad peaks appeared at 28.2˚, 29.5˚, and 32.5˚. These peaks were different from several peaks of the JCPDS41-1089 of Nd2O3, the JCPDS83-2035 of Nd(OH)3, and the JCPDS85-1198 of neodymium oxy-chloride, NdOCl, and were thought to be depended on the compound generated by a reaction of SUS304 compositions with Nd2O3 or CCl4. This result indicated that Nd2O3 was not chlorinated by a milling with CCl4 in a SUS304 pot and balls.
In the XRD pattern of the product obtained by a milling at 500 rpm for 1 h in a zirconia pot with 10 mmφ × 16 balls of run-2, new broad peaks appeared as shown in Figure 2(c). These peaks were corresponding to peaks of the JCPDS85-1198 of NdOCl at 13.0˚, 25.7˚, 31.4˚, and 34.5˚. The color of this product was light blue near the mauve color of neodymium tri-chloride, NdCl3, and the color of NdOCl single crystals was pale purple [18]. Thus, it was thought that the product obtained by a milling of Nd2O3 with CCl4 in a zirconia pot and balls was NdOCl. However, the peak at 30.8˚ of Nd2O3 was observed in this XRD pattern, and the chlorination from Nd2O3 with CCl4 was thought not to complete by a milling for 1 h. Therefore, it was confirmed the possibility that NdOCl was synthesized from Nd2O3 with CCl4 by using a planetary ball mill in a zirconia pot and balls, though the Nd2O3 remained in the product.
Moreover, the XRD pattern of the product obtained by a milling at 500 rpm in a tungsten carbide pot with 10 mmφ × 8 balls of run-3 was not observed new peaks as shown in Figure 2(d), though the peaks of Nd2O3 became extremely gradual. And this product did not change color and kept a light blue color of Nd2O3. From the above results, it was thought that the milling time for 1 h was not enough to chlorinate from Nd2O3 with CCl4 to NdOCl in a tungsten carbide pot and balls.
The XRD patterns and Raman spectra of products by mechanochemical experiments of run-4 to run-19 are shown in Figures 4 and 5, respectively.
3.2. Possibility of Chlorination to NdOCl by Milling in Zirconia or Tungsten Carbide Pot
As described in the above section, the milling for 1 h in a tungsten carbide pot with 10 mmφ × 8 balls was not enough to chlorinate to NdOCl. According to the computer simulation in a planetary ball milling, the specific impact energy in a milling is proportional to the mass of balls [8,9]. If the NdOCl amount in a product obtained by a milling was proportional to the impact energy, this NdOCl amount by a milling in a tungsten carbide pot should be more than that in a zirconia pot because the density of tungsten carbide is 2.5 times of zirconia. On the same milling conditions, it was presumed that the NdOCl amount in a product obtained in a tungsten carbide pot increased more than that in a zirconia pot.
In order to confirm the possibility of chlorination from Nd2O3 with CCl4 in a tungsten carbide pot and to compare a product obtained in a zirconia pot with that in a tungsten carbide pot, the mechanochemical experiments were conducted in the same condition by a milling at 500
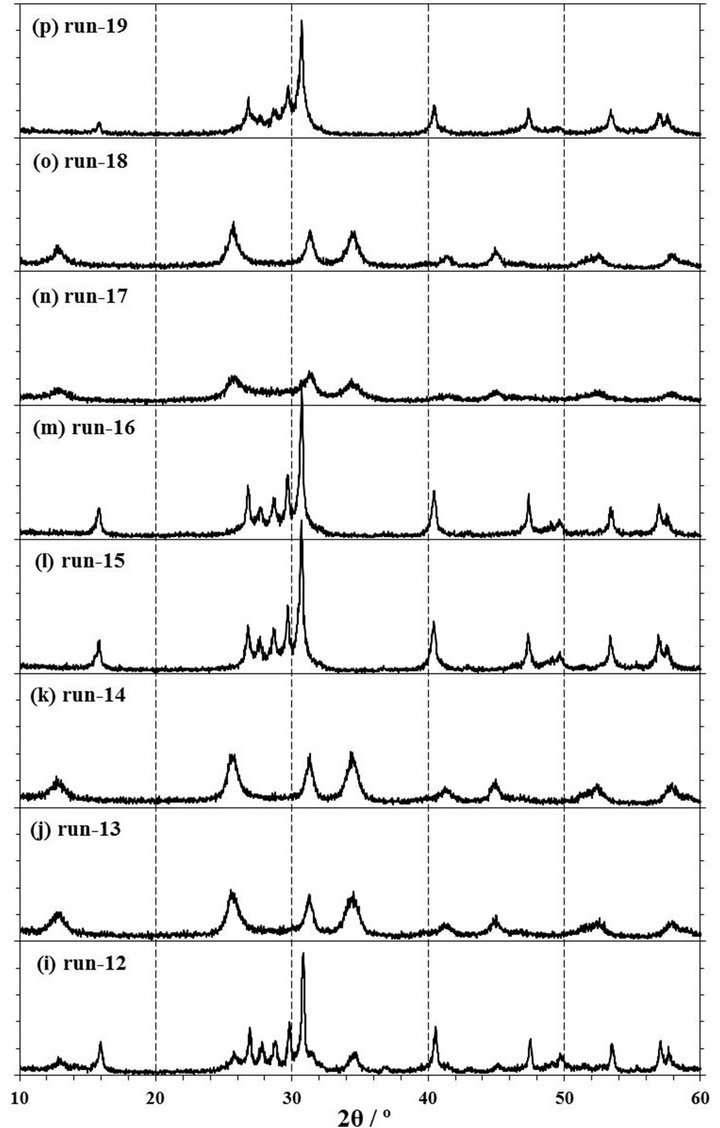
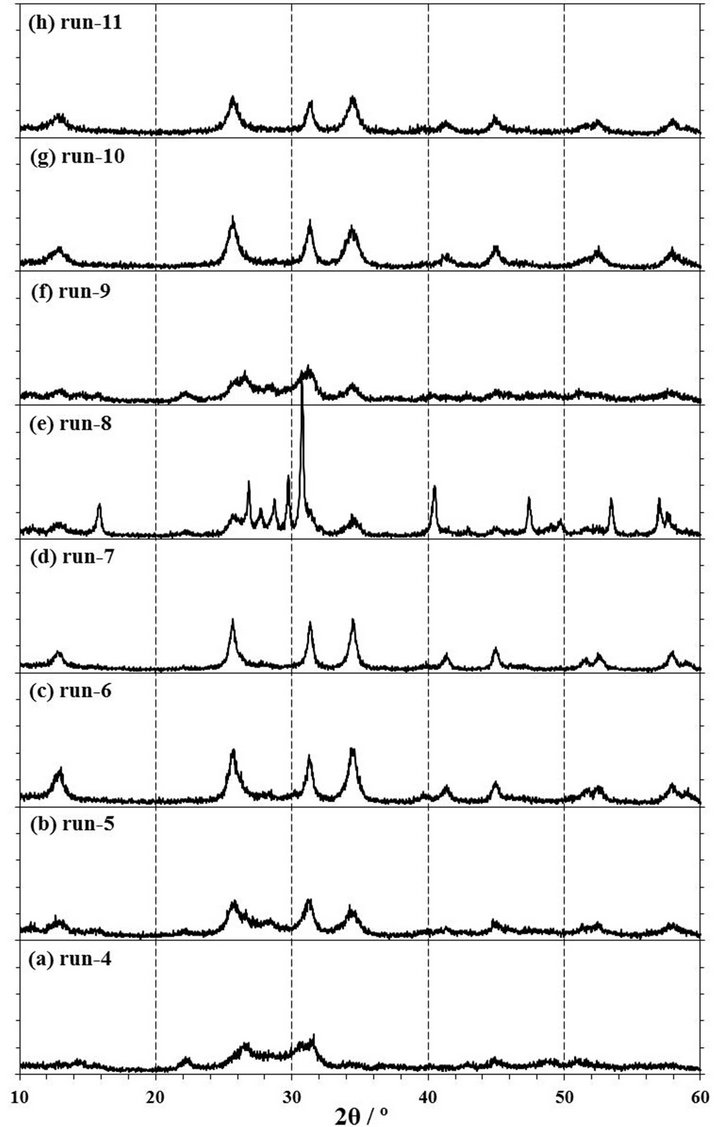
Figure 4. XRD patterns of products obtained by mechanochemical experiments of run-4 to run-19. The experimental conditions are described in Table 1. 1 span of Y-axis is 100 cps.
rpm for 4 h in a zirconia (run-9) or tungsten carbide (run-18) pot with each 10 mmφ × 8 balls. The XRD patterns of these products are shown in Figures 4(f) and (o). It was observed that the XRD patterns of both products in a zirconia and tungsten carbide pot were contained the clear peaks of NdOCl at 13.0˚, 25.7˚, 31.4˚, and 34.5˚, suggesting that NdOCl was formed from Nd2O3 with CCl4 in a zirconia or tungsten carbide pot with 8 balls. Moreover, when the Raman spectra of those products as shown the in Figures 5(f) and (o) were measured, these products were observed the Raman shifts at 118, 176, 344, and 453 cm−1. The Raman shifts of tetragonal NdOCl are 118, 183, 352, and 465 cm−1 and these products were considered to be NdOCl [19]. As a reason to which measured peak positions became lower than reference values [19], since the Raman peak positions of RuO2 shifted to lower wavenumber by residual stress [20-22], it was presumed that stress remained in the products even after a milling operation.
We found that NdOCl was synthesized in a tungsten carbide or zirconia pot with balls, and that the generation of NdOCl by a milling in a tungsten carbide pot was easier than that in a zirconia pot. Therefore the higher impact energy is needed to chlorinate to NdOCl, and the NdOCl amount in the product increases with an increase of the impact energy. In other words, the use of pot and balls made of a heavy density material is effective to obtain higher impact energy for the chlorination to NdOCl by a planetary ball milling. Because the mechanical load to the milling equipment increases with an increase of the impact energy, it is necessary to select a milling condition below the limitation of the equipment. In the present study by using a tungsten carbide pot and balls, the number of ball was performed by 8 balls to prevent the failure of this planetary ball mill.
3.3. Influence on Chlorination to NdOCl by Milling Time
To confirm the effect of the chlorination to NdOCl by
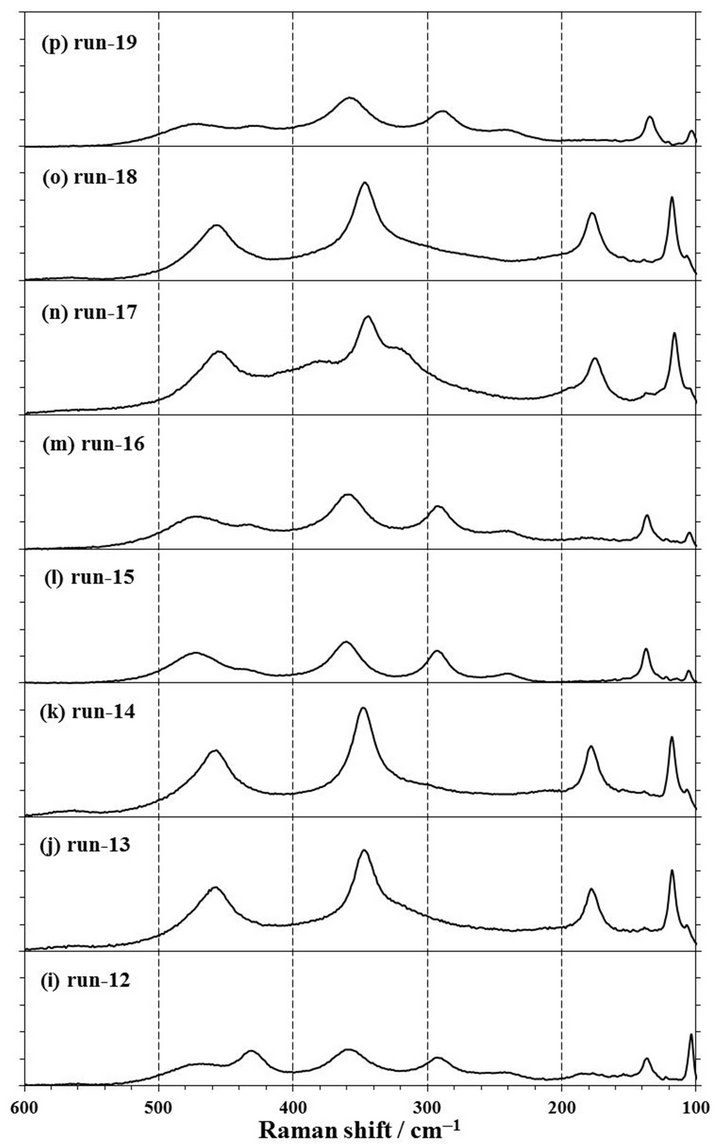
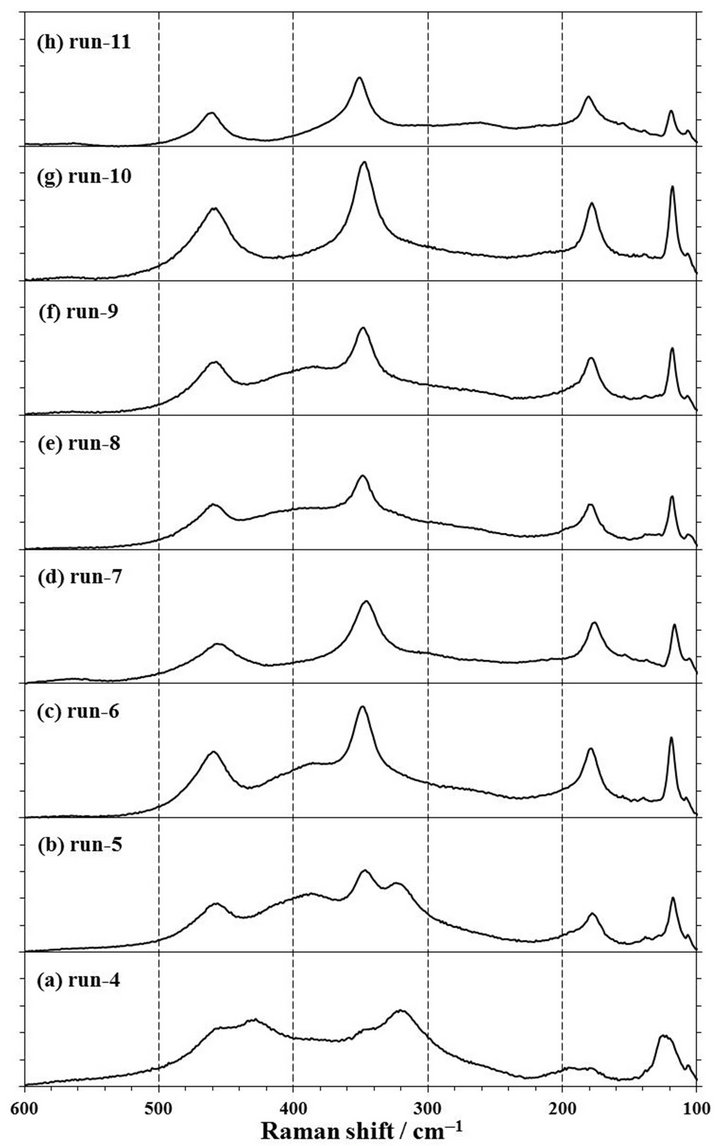
Figure 5. Raman spectra of products obtained by mechanochemical experiments of run-4 to run-19. The experimental conditions are described in Table 1. 1 span of Y-axis is 25 counts.
extending the milling time, the milling operation was carried out for the different milling time of 0.5, 1, 2, and 4 h in a zirconia pot with 10 mmφ × 16 balls or a tungsten carbide pot with 10 mmφ × 8 balls. As shown in Figures 4 (a)-(d), it can be confirmed that NdOCl peaks of XRD patterns of products obtained in a zirconia pot became sharper with an extension of the milling time and that Nd2O3 peaks in the XRD patterns disappeared simultaneously. The pattern of run-4 by a milling for 0.5 h as shown in Figure 4(a) was not observed any clear NdOCl peak, and that of run-5 by a milling for 1 h as shown in Figure 4(b) was confirmed main NdOCl peaks at 13.0˚, 25.7˚, 31.4˚, and 34.5˚. When the milling time was extended to 2 h of run-6, these NdOCl peaks were observed more clearly as shown in Figure 4(c). However, the peaks of run-6 did not have a difference with those of 4 h milling of run-7 as shown in Figure 4(d). As for the Raman spectra shown in Figures 5(a)-(d), the NdOCl peaks were observed in all the products. And those peaks in products increased with an expansion of the milling time until 2 h, but those peaks of the product by a milling time for 4 h of run-7 decreased as shown in Figure 5(d). Since the synthesized NdOCl crystals were ground by milling in planetary ball mill, it is presumed that the NdOCl peaks of Raman spectra decreased with excessive milling time. These results of XRD patterns and Raman spectra were thought to indicate that the chlorination to NdOCl was almost completed until 2 h of the milling time in a zirconia pot with 10 mmφ × 16 balls.
In another case of using a tungsten carbide pot with 10 mmφ × 8 balls, NdOCl peaks of XRD patterns of products increased with extending the milling time of 1, 2, and 4 h as shown in Figures 2(d), 4(n) and (o). Also NdOCl peaks of Raman spectra of the products became sharper with an expansion of milling time as shown in Figures 3(c), 5(n) and (o). These results indicated that the chlorination to NdOCl from Nd2O3 with CCl4 was not completed by a milling for 2 h. In other words, it can be said that the milling time more than 4 h is needed for the chlorination to NdOCl when a tungsten carbide pot with 10 mmφ × 8 balls is used.
As these results, it was confirmed that an extension of the milling time was effective in completing the chlorination from Nd2O3 to NdOCl by using a planetary ball mill.
3.4. Influence on Chlorination to NdOCl by Molar Ratio of CCl4/Nd2O3
As described in above sections, it was found that NdOCl was produced from Nd2O3 with CCl4 by using a planetary ball mill. In the present study, the standard molar ratio of CCl4/Nd2O3 was selected to be 3.47. If NdOCl could generate by the Reaction (1), the molar ratio would be enough with CCl4/Nd2O3 = 0.5. Since CCl4 has the low boiling point, the initial amount of CCl4 needs to take carrying out an evaporation loss of CCl4 from a pot during a milling operation. However, when CCl4 is added superfluously into a pot, it is necessary to remove CCl4 which remained in the product.
 (1)
(1)
To confirm to the suitable molar ratio of CCl4/Nd2O3, the mechanochemical experiments were carried out by a milling at 500 rpm for 2 and 4 h in a zirconia pot with 10 mmφ × 16 balls in the condition of CCl4/Nd2O3 = 1.73, 3.47, and 6.93. At the molar ratio of CCl4/Nd2O3 = 1.73 by a milling for 2 h of run-12, there were large peaks of the Nd2O3 reagent and small NdOCl peaks in the XRD pattern of the product as shown in Figure 4(i). Also in the Raman spectra of this product of run-12 as shown in Figure 5(i), NdOCl peaks were small and Nd2O3 peaks were mainly observed. On the other hand, NdOCl peaks were observed in the XRD patterns and Raman spectra of the products by a milling for 2 h in the molar ratio of CCl4/Nd2O3 = 3.47 of run-6 and 6.93 of run-13 as shown in Figures 4(c), (j), 5(c) and (j), respectively. The difference in the XRD patterns of products by a milling in the molar ratio of CCl4/Nd2O3 = 3.47 of run-6 and 6.93 of run-13 was not observed as shown in Figures 4(c) and (j), but the NdOCl peaks of the Raman spectrum in the molar ratio of CCl4/Nd2O3= 3.47 of run-6 became slightly higher than those in CCl4/Nd2O3= 6.93 of run-13 as shown in Figures 5(c) and (j). Since the balls were thought to slide on the pot inner face and the NdOCl synthesis did not advance when there was too much CCl4 liquid volume in a case of run-13, it is presumed that the NdOCl peaks of the Raman spectra did not increase with increasing of the molar ratio of CCl4/Nd2O3.
When the milling was operated for 4 h, the XRD pattern in the molar ratio of CCl4/Nd2O3 = 3.47 of run-7 is similar to that of CCl4/Nd2O3= 6.93 of run-14 as shown in Figures 4(d) and (k). However, as for the Raman spectra as shown in Figures 5(d) and (k), the NdOCl peaks in the molar ratio of CCl4/Nd2O3 = 6.93 was slightly higher than those in CCl4/Nd2O3 = 3.47. At an initial state of milling operation in a case of too much CCl4 liquid volume of run-14, the balls were thought to slide on the inner wall of the pot as well as run-13, but the NdOCl synthesis could advance with decreasing CCl4 liquid volume by the vaporization. In the molar ratio of CCl4/Nd2O3 = 3.47 of run-7, the NdOCl synthesis advanced, but it is presumed that the NdOCl peaks of Raman spectra decreased because the synthesized NdOCl crystals at the Reaction (1) were ground by the continued milling operation in planetary ball mill.
Therefore, it can be said that the molar ratio of CCl4/ Nd2O3 = 3.47 is enough to synthesize NdOCl from 0.4 g of Nd2O3 with CCl4 by a milling at 500 rpm for 2 or 4 h in a zirconia pot and balls. Since the initial amount of CCl4 against Nd2O3 fully existed in condition of the molar ratio of CCl4/Nd2O3 = 6.93, the chlorination to NdCl3 by the reaction (2) had been expected. But the generation of NdCl3 was not confirmed in these experiments.
 (2)
(2)
3.5. Influence on Chlorination to NdOCl by Number of Ball
From results described in above sections, it was clear that the chlorination to NdOCl requires the sufficient impact energy and the number of collision of a ball against other balls or the pot wall. An extension of the milling time described in the above section means an increase of the number of collision, and an increase of the number of ball was also expectable as a method of increasing the number of collision.
To confirm the influence on the chlorination to NdOCl by the number of ball, the mechanochemical experiments were carried out by a milling at 500 rpm for 2 h in a zirconia pot with 10 mmφ × 8, 12, 16, and 24 balls. In a case of 8 balls, the Nd2O3 peaks of a XRD pattern of run-8 were observed as shown in Figure 4(e). When the number of ball was more than 12 or more of run-10, -6, and -11, the Nd2O3 peaks disappeared and the NdOCl peaks were observed as shown in Figures 4(c), (g) and (h). In the other words, it was confirmed that the number of ball was required 12 or more for the chlorination to NdOCl from Nd2O3 with CCl4 by a milling at 500 rpm in a zirconia pot. Comparing these patterns in Figures 4(c), (e), (g) and (h), the pattern by a milling with 16 balls of run-6 was the sharpest. In the case of putting 24 balls in a pot, it was presumed that the motion of balls in a pot was restrained and the number of collision decreased in this milling condition. Consequently, it is considered that the XRD pattern with 24 balls was not clearer than that with 16 balls.
As for the Raman spectra, the NdOCl peaks were observed in all the products. Comparing these spectra as shown in Figures 5(c), (e), (g) and (h), the NdOCl peaks in a product of run-10 in a case of putting 12 balls were higher than those of run-8 in a case of 8 balls, and were similar to those of run-6 in a case of 16 balls. But those peaks in the product of run-11 in a case of 24 balls as shown in Figure 5(h) were lower than those in 12 or 16 balls of run-6. The reason to which the NdOCl peaks decreased with increasing to 24 balls was presumed that the motion of balls in a pot was restrained and the number of collision decreased in this milling condition.
Therefore, it was found that the number of collision increased with an increase of the number of ball on the conditions which the number of ball was 16 or less, and it is thought that 16 balls are suitable in this milling condition.
3.6. Chlorination Effect by Revolution Speed
The revolution speed of planetary disk is one of important parameters of the impact energy in operating a planetary ball mill [8,9]. The impact energy increases with an increase of the revolution speed, but the failure risk of the mill increases with an increase of the impact energy. To decrease this risk, additional experiments of run-15, -16 in a zirconia pot and run-19 in a tungsten carbide pot were carried out in the condition of the revolution speed at 250 rpm. The conditions of run-15, -16, and -19 except the revolution speed were similar to those of run-5, -6, and -17, respectively.
As results shown in Figures 4(l), (m) and (p), all XRD patterns of products of run-15, -16, and -19 by milling at 250 rpm were similar to that of a source material of Nd2O3, and any peak of NdOCl were not observed. As for the Raman spectra in Figure 5(l), (m) and (p), the NdOCl peaks of products of run-15, -16, and -19 by milling at 250 rpm were smaller than those of products of run-5, -6, and -17 at 500 rpm as shown in Figures 5(b), (c) and (n). And the color of these products was not changed from a light blue of Nd2O3. According to the simulation in a planetary ball milling [8], the impact energy was proportional to the square of the collision speed of balls, and the relation between this collision speed and the revolution speed had a correlation near proportion accurately though was not proportion. Therefore, it was confirmed that the chlorination to NdOCl from Nd2O3 with CCl4 was not able to perform by a milling in the revolution speed at 250 rpm, which the impact energy was one-quarter of that at 500 rpm.
3.7. Thermodynamic Consideration for Chlorination of Nd2O3 with CCl4
To understand the chlorination to NdCl3 from Nd2O3 with CCl4 described in above sections, their thermodynamic reaction was examined using a thermodynamic data base of the MALT [23].
Figure 6 shows the temperature dependence of the Gibbs free energy changes, ΔGº, of the formation of NdOCl and NdCl3 from Nd2O3 with CCl4, and NdCl3 from NdOCl with CCl4. The ΔGº of formation of both NdOCl and NdCl3 from Nd2O3 are negative values in the temperature range from 100˚C to 900˚C. The ΔGº of NdOCl is more negative than that of NdCl3. In other words, the formation of NdOCl should be caused easily thermodynamically than that of NdCl3. However, when the Nd2O3 powder reagent immersed in CCl4 liquid for 24 h at room temperature and the XRD pattern of the reagent after the immersion was measured, the XRD pattern was similar to the initial Nd2O3 reagent. Therefore, the generation to NdOCl from Nd2O3 with CCl4 is thought to be very slow at room temperature.
From the results estimated by the MALT in calculating condition with total pressure of 1 atm, the potential diagram of the Nd-O2-Cl2 system at 25˚C, 100˚C, and 200˚C was drawn in Figure 7. According to the mechanical alloying studies [4,5], the temperature of a ball surface at a collision was estimated to be at 200˚C - 500˚C. And the decomposition pressures of a source reagent of CCl4 to Cl2 and a by-product material of CO2 to O2 at 25˚C, 100˚C, and 200˚C are showed as lines in Figure 7. Nd is chlorinated to NdCl3 with increasing a partial pressure of Cl2, and oxidized to Nd2O3 with increasing a partial pressure of O2. But in our mechanochemical experiments, NdOCl was obtained in an atmosphere condition of
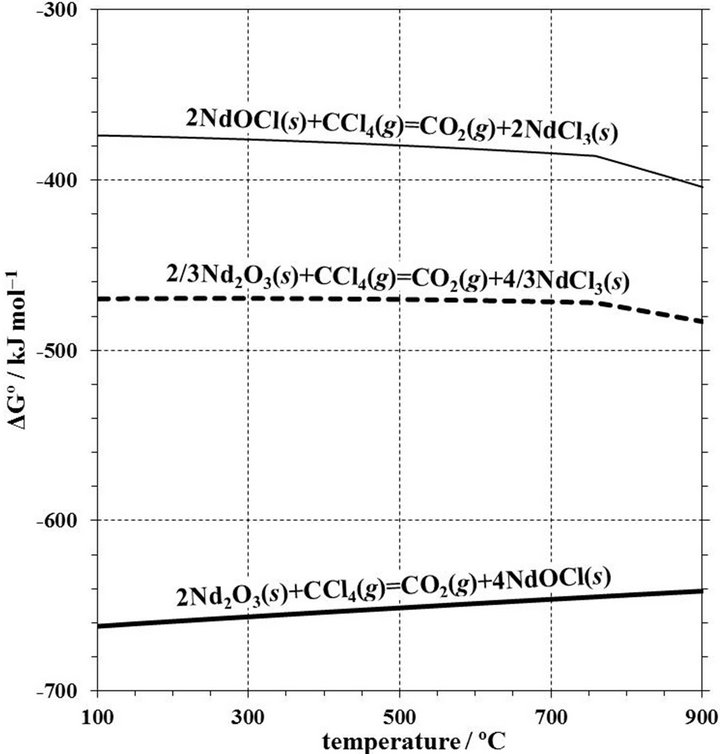
Figure 6. Temperature dependence of the Gibbs free energy changes, ΔGº, from Nd2O3 with CCl4 to NdOCl or NdCl3, and from NdOCl with CCl4 to NdCl3.
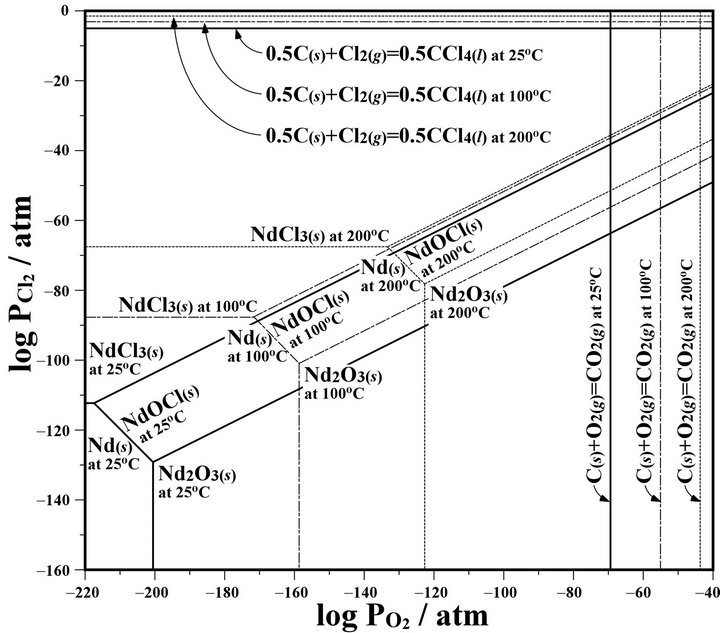
Figure 7. Potential diagram of the Nd-O2-Cl2 system at different temperatures. The data at 25˚C, 100˚C, and 200˚C are given as solid, chain and dotted lines, respectively.
coexisting with Cl2 and O2. This NdOCl is presumed to be an intermediate product to NdCl3. As a reason why these products were NdOCl, the generation to NdCl3 from NdOCl with CCl4 is thought to be very slow in our experimental conditions as well as the generation to NdOCl from Nd2O3 with CCl4 at room temperature. On the other hand, if the generation to NdCl3 from NdOCl would not be slow, it is presumed that the partial pressure of Cl2 in a pot did not reach to the processing condition of NdCl3 from the potential diagram of the Nd-O2-Cl2 system as shown Figure 7. That is, in order to enable generation of NdCl3 from Nd2O3 by using a planetary ball mill, it is necessary to perform either of an increase of the partial pressure of Cl2 in the pot or a large extension of the milling time.
3.8. Structural Change of Nd2O3 and NdOCl Powder by Milling Treatment
As described in above sections, NdCl3 was not synthesized from Nd2O3 with CCl4, but NdOCl could be obtained easily at room temperature by using a planetary ball mill. The chlorination from Nd2O3 to NdOCl was very slow when Nd2O3 was immersed in CCl4 liquid, and it was confirmed that this chlorination progressed with expansion of the milling time by using a planetary ball mill. In the early stage of a milling operation, it was thought that the pulverization of Nd2O3 reagent progressed before chlorination of from Nd2O3 to NdOCl.
To confirm the structural change of Nd2O3 and NdOCl powders by using a planetary ball mill, the raw Nd2O3 reagent, dried Nd2O3 and prepared NdOCl powders were milled in each a zirconia pot with 16 balls for 0.5 h with revolution speed at 500 rpm. The dry treatment of Nd2O3 powder was heated the raw Nd2O3 reagent in air at 700˚C for 1 h, and the preparation of NdOCl powder was raw Nd2O3 and NdCl3 reagents mixture and heated in air at 300˚C for 5 h. Figures 8 and 9 show the XRD patterns and Raman spectra of the powders before and after a milling, respectively. Here, the XRD patterns were measured in an irradiation condition at 40 kV and 40 mA, and the Raman spectroscopy were performed on the same conditions described in above sections.
The XRD patterns of the milled Nd2O3 and NdOCl powders as shown in Figures 8(b), (c) and (e) were smaller and broader than those of the powders before the milling treatment, respectively. The large peaks of Nd(OH)3 were existed in the XRD pattern of a raw Nd2O3 reagent as shown in Figure 2(a), and the pattern of Nd2O3 powder immediately after the dry treatment hardly observed Nd(OH)3 peaks as shown in Figure 8(a). From the weight loss of raw Nd2O3 reagent by the dry treatment, the amount of Nd(OH)3 contained in the raw reagent was estimated as 48.6 wt%. Comparing the XRD patterns of milled Nd2O3 powders as shown in Figures 8(b) and (c), the peaks of NdOOH at 16˚ and 22˚ were observed in the milled raw Nd2O3 reagent containing Nd(OH)3. As for the generation of NdOOH, it has been
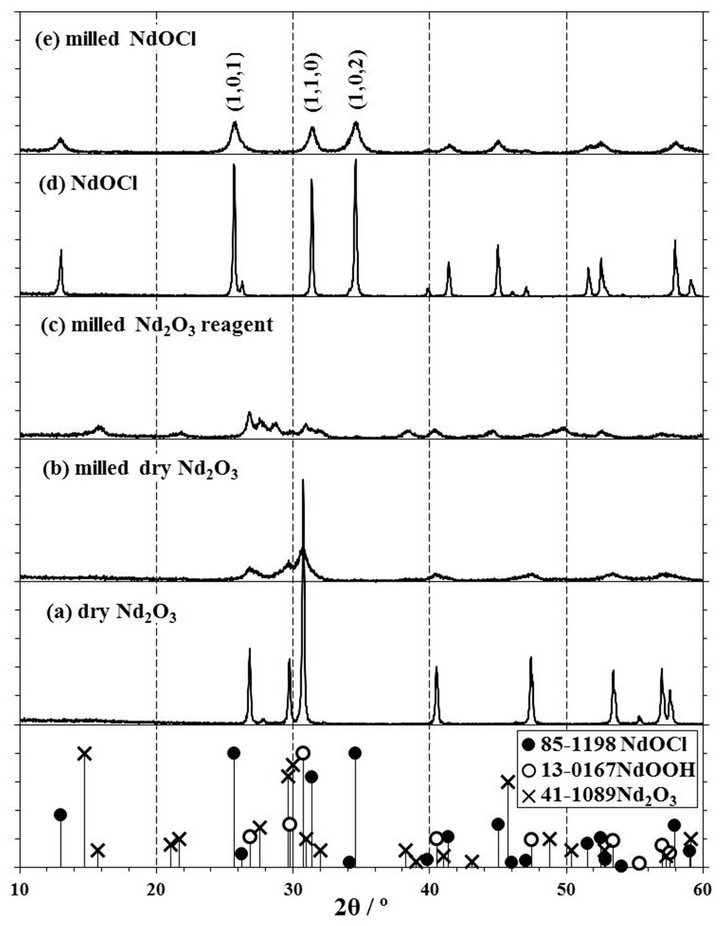
Figure 8. XRD patterns of (a) dry Nd2O3, (b) milled dry Nd2O3, (c) milled Nd2O3 reagent without dry treatment, (d) NdOCl, and (e) milled NdOCl. The milling condition of (b), (c), and (e) is in zirconia pot with 10 mmφ × 16 balls for 2 h at 500 rpm. 1 span of Y-axis is 100 cps.
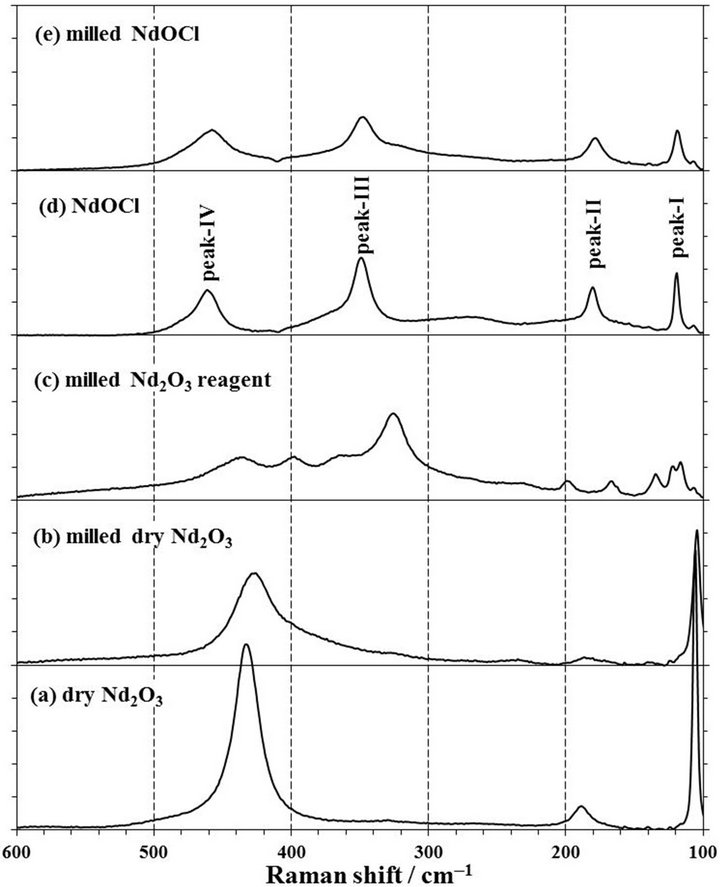
Figure 9. Raman spectra of (a) dry Nd2O3, (b) milled dry Nd2O3, (c) milled Nd2O3 reagent without dry treatment, (d) NdOCl, and (e) milled NdOCl. The milling condition of (b), (c), and (e) is in zirconia pot with 10 mmφ × 16 balls for 2 h at 500 rpm. 1 span of Y-axis is 25 counts.
reported that NdOOH crystals deposited on the cathode surface by an electrolysis operation in the NaOH-0.3 mol% Nd(OH)3 mixture melt at 400˚C [24], and that NdOOH was synthesized from Nd2O3-H2O system by keeping a pressure of 20 MPa at 500˚C for 120 h [25]. In the XRD patterns of mechanochemical products obtained by a milling, since these peaks at 16˚ and 22˚ were observed small as shown in Figures 2(c), (d), 4(a), (b), (e) and (f), it was thought that NdOOH generated from Nd(OH)3 contained in the Nd2O3 reagent. The NdOOH was chlorinated with CCl4 to NdOCl with the chlorination of Nd2O3 to NdOCl by a mechanochemical reaction, since the peaks of NdOOH was not observed in the XRD patterns of products obtained on sufficient chlorination conditions. Since it was suggested according to the sulfurization of TiO2 or Ti(OH)4 with CS2 to Ti3S4 that the reaction temperature of Ti(OH)4 was lower than that of TiO2 [26], the chlorination of Nd(OH)3 with CCl4 to NdOCl might be easier to be chlorinated than that of Nd2O3.
The Raman spectra of the milled Nd2O3 and NdOCl powders show in Figures 9(b) and (e), and the large difference were not observed in comparison with those of the powders before the milling treatment. However, in the milled raw Nd2O3 reagent which a generation of NdOOH was confirmed by a XRD analysis, the peak was observed at 325 cm−1 in the Raman spectrum as shown in Figure 9(c). This peak was considered to be identified the peak of NdOOH at 339 cm−1 [27], and it was presumed that the peak shifted by a residual stress after the milling treatment. This peak at 325 cm−1 was also observed also in the spectra of mechanochemical products as shown in Figures 3(b), (c), 5(a), (b) and (n). On the other hand, the Nd2O3 peak of the dry Nd2O3 powder was observed a shift to 425 cm−1 from 433 cm−1 after the milling treatment as shown in Figure 9(b).
As for the peak shift of Raman spectra, the most of NdOCl spectrum peaks in the products obtained in our mechanochemical experiments were shifted to lower wavenumbers than those of tetragonal NdOCl crystals [19], and they were considered to be influence of the residual stress by a milling as described above sections. According to the Raman spectroscopy measurements of Cr2O3 thin films [28], Y2O3-ZrO2 barrier coatings [29], epitaxial GaN layers [30], and Al2O3 single crystals [31], in the state where the stress was applied, the redshift which the peak shifted to low wavenumbers was based on the tensile stress, and the tensile stress remained in the surface of products which obtained by using a planetary ball mill. Therefore, the large compressive force of the level on which the tensile stress remained in the products was loaded on particles during our mechanochemical experiments, and contributed to progress of the chlorination to NdOCl from Nd2O3 with CCl4.
3.9. Relevant Milling Condition for Chlorination to NdOCl
In the present study, most mechanochemical experiments were carried out using a zirconia pot and balls in various milling conditions, such as milling time, molar ratio of CCl4/Nd2O3, number of ball, and revolution speed. It was confirmed in the Section 3.6 that the chlorination to NdOCl progressed with an acceleration of the revolution speed.
From the XRD patterns and Raman spectra of mechanochemical products, we examined the relevant milling time, molar ratio of CCl4/Nd2O3, and number of ball for the effective chlorination to NdOCl from Nd2O3 with CCl4. The NdOCl peaks in XRD patterns of products of run-5, -6, -7, -10, -11, -12, -13, and -14 exists as shown in Figure 4, and the NdOCl peaks in their Raman spectra from run-4 to run-14 except run-12 exists in Figure 5. Although the relevant conditions are that all added Nd2O3 powders in a pot react with CCl4, it is also necessary to not be worsened the crystal structure of products and to decrease the residual stress of products by milling operation. The crystal structure is worsened to fine particles by a milling operation, and the peaks of XRD pattern shows broad as shown in Figure 8. When the residual tensile stress increases, the characteristic Raman peak shifts to lower wavenumber as described in section 3.8. Thus, the peak width in a XRD pattern and the peak shift of a Raman spectrum can be used as the index which examines a relevant condition. The peak width in a XRD pattern was estimated using a full width at half maximum, FWHM, of (1,0,1), (1,1,0), and (1,0,2) peaks. The Raman shift, Δν, was calculated the deference with the wavenumber in Raman peak-I at 119.4 cm−1, peak-II at 180.7 cm−1, peak-III at 349.1 cm−1, and peak-IV at 461.0 cm−1 of the dry NdOCl powder as shown in Figure 9(d).
Figure 10 shows the change of FWHM of XRD peaks and Δν of Raman peaks by the milling time in the case of 10 mmφ × 16 balls and molar ratio of CCl4/Nd2O3 =3.47 in a zirconia pot. The FWHM values of XRD peaks decrease with an increase of milling time, and the Δν values of Raman peaks in 2 h is smaller than those in 1 or 4 h. Since the difference of FWHM values in between 2 and 4 h are small, it is thought that the chlorination to NdOCl was completed in 2 h and then it is presumed that the distortion was given to the produced NdOCl powder by milling operation. The change of FWHM and Δν by the molar ratio of CCl4/Nd2O3 in the case of 10 mmφ × 16 balls in a zirconia pot for 2 h is shown in Figure 11. When the molar ratio of CCl4/Nd2O3 increased, the FWHM values increased and the Δν values were more negative. As described in the section 3.4, it is thought that the molar ratio of CCl4/Nd2O3 = 3.47 was enough to synthesize NdOCl. As for the change of FWHM and Δν by the number of 10 mmφ balls in a zirconia pot in the milling
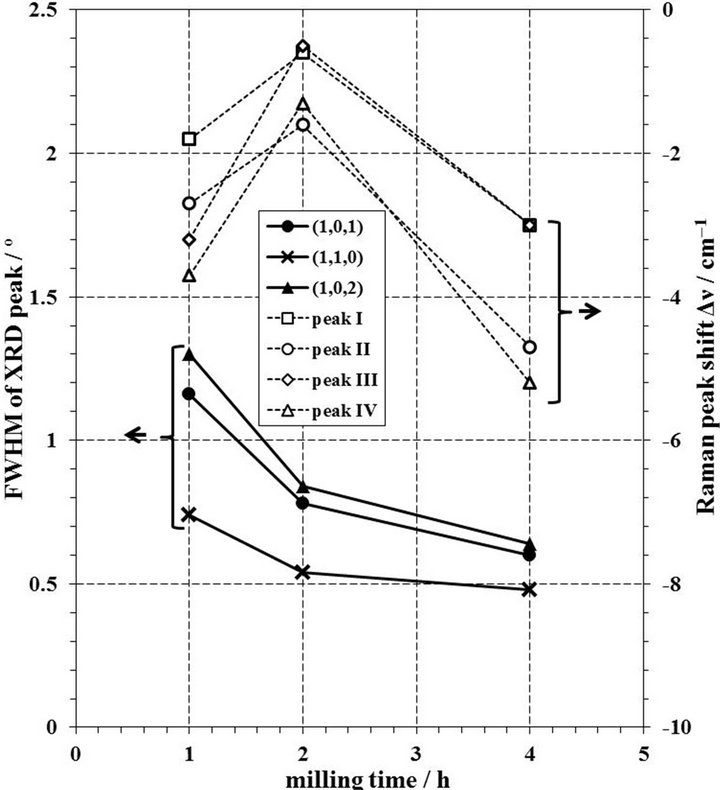
Figure 10. Change of FWHM of XRD peaks and Δν of Raman peaks by milling time in case of 10 mmφ × 16 balls and molar ratio of CCl4/Nd2O3 = 3.47 in a zirconia pot.
case of the molar ratio of CCl4/Nd2O3 = 3.47 for 2 h, the FWHM and Δν values by milling with 16 balls are smaller than the other conditions as shown in Figure 12. However, the Δν shows scattered values in milling with 8 or 24 balls. This reason is thought that the chlorination to NdOCl was not completed, because the milling with 8 balls was not enough to obtain the required energy for chlorination to NdOCl and the milling with 24 balls restrained the motion of balls in a pot. Therefore, the relevant milling conditions for chlorination to NdOCl from 0.4 g of Nd2O3 with CCl4 in a zirconia pot at 500 rpm is selected that the milling time is 2 h, the molar ratio of CCl4/Nd2O3 is 3.47, and the number of balls is 16.
4. Conclusions
In the present study, the chlorination from Nd2O3 with CCl4 was carried out using a planetary ball mill, and the products were estimated by the XRD analysis and Raman spectroscopy. The obtained results are as follows.
It was confirmed that the chlorination to NdOCl from Nd2O3 with CCl4 was advanced at room temperature by the mechanochemical method. However, NdCl3 could not be synthesized in this mechanochemical study. The products obtained by a milling in a zirconia or tungsten carbide pot were pale purple colored NdOCl. But NdOCl was not produced by a milling in a SUS304 pot. An extension of the milling time, an increase of the number of ball, an acceleration of the revolution speed, and the selection of the pot made of a high density material were
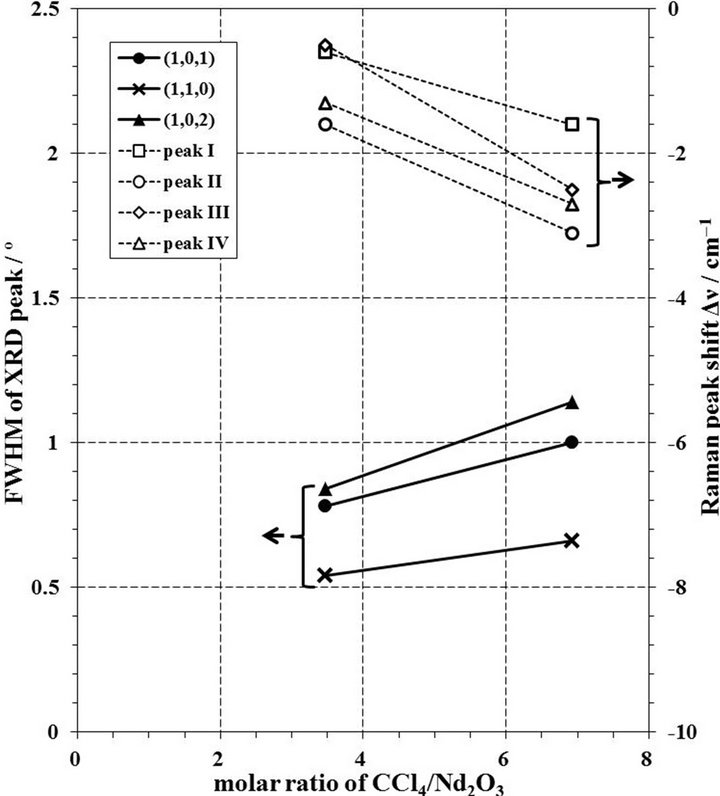
Figure 11. Change of FWHM of XRD peaks and Δν of Raman peaks by molar ratio of CCl4/Nd2O3 in case of 10 mmφ × 16 balls for 2 h milling in a zirconia pot.
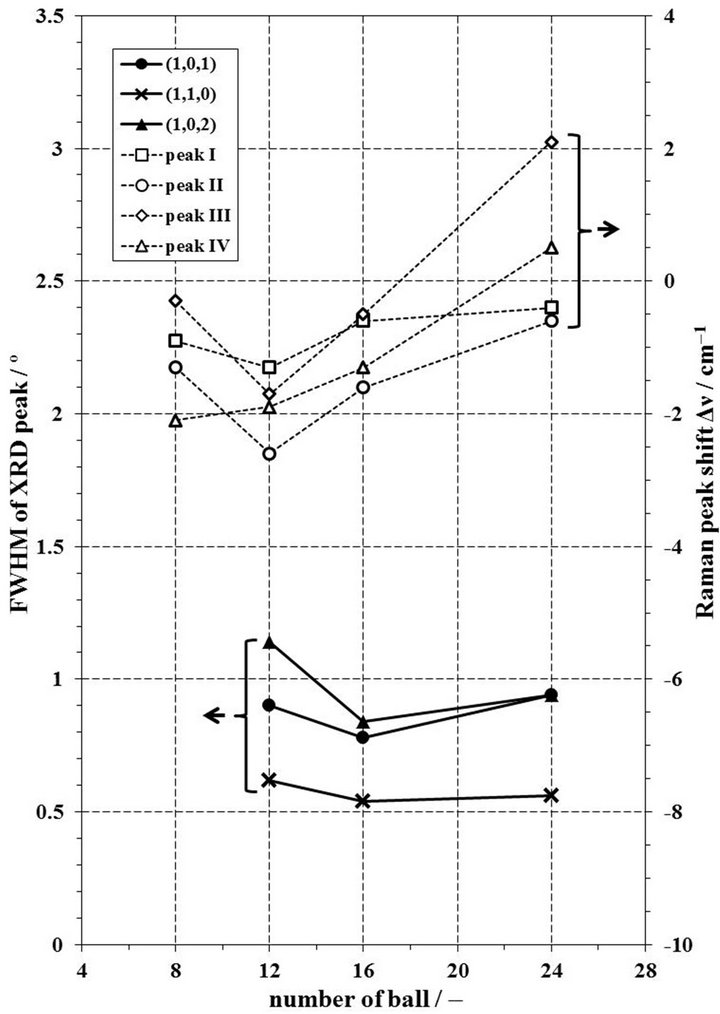
Figure 12. Change of FWHM of XRD peaks and Δν of Raman peaks by number of 10 mmφ balls in case of molar ratio of CCl4/Nd2O3 for 2 h milling in a zirconia pot.
effective for the chlorination to NdOCl from Nd2O3 with CCl4. NdOOH was generated from Nd(OH)3 of impurity contained in Nd2O3 reagent by a milling, and this NdOOH was chlorinated with CCl4 to NdOCl by a mechanochemical reaction. It was confirmed that tensile stress remained in the milled powder by using a planetary ball mill.
REFERENCES
- T. Nishimura, T. Koyama, M. Iizuka and H. Tanaka, “Development of an Environmentally Benign Reprocessing Technology—Pyrometallurgical Reprocessing Technology,” Progress in Nuclear Energy, Vol. 32, No. 3-4, 1998, pp. 381-387. doi:10.1016/S0149-1970(97)00032-2
- Y. Sakamura, T. Inoue, T. Iwai and H. Moriyama, “Chlorination of UO2, PuO2 and Rare Earth Oxides Using ZrCl4 in LiCl-KCl Eutectic Melt,” Journal of Nuclear Materials, Vol. 340, No. 1, 2005, pp. 39-51. doi:10.1016/j.jnucmat.2004.11.002
- N. Sato, H. Mohamad, J. Kano, A. Kirishima and F. Saito, “Low Temperature Sulfurization of Nd2O3 by Mechanochemical Method with CS2,” Journal of MMIJ, Vol. 126, 2010, pp. 445-449 (in Japanese). doi:10.2473/journalofmmij.126.445
- A. K. Bhattacharya and E. Arzt, “Temperature Rise during Mechanical Alloying,” Scripta Metallurgica et Materialia, Vo. 27, No. 6, 1992, pp. 749-754. doi:10.1016/0956-716X(92)90500-E
- M. Abdellaoui and E. Gaffet, “A Mathematical and Experimental Dynamical Phase Diagram for Ball-Milled Ni10Zr7,” Journal of Alloys and Compounds, Vol. 209, No. 1-2, 1994, pp. 351-361. doi.:10.1016/0925-8388(94)91124-X
- M. Abdellaoui and E. Gaffet, “The Physics of Mechanical Alloying in a Planetary Ball Mill: Mathematical Treatment,” Scripta Metallurgica et Materialia, Vol. 43, No. 3, 1995, pp. 1087-1098. doi:10.1016/0956-7151(95)92625-7
- Y.-S. Kwon, K. B. Gerasimov and S.-K. Yoon, “Ball Temperature during Mechanical Alloying in Planetary Mill,” Journal of Alloys and Compounds, Vol. 346, No. 1, 2002, pp. 276-281. doi:10.1016/S0925-8388(02)00512-1
- J. Kano, H. Mio and F. Saito, “Correlation of Size Reduction Rate of Inorganic Materials with Impact Energy of Balls in Planetary Ball Milling,” Journal of Chemical Engineering of Japan, Vo. 32, No. 4, 1999, pp. 445-448. doi:10.1252/jcej.32.445
- H. Mio, J. Kano, F. Saito and K. Kaneko, “Effects of Rotational Direction and Rotation-to-Revolution Speed Ratio in Planetary Ball Milling,” Materials Science and Engineering: A, Vol. 332, No. 1-2, 2002, pp. 75-80. doi:10.1016/S0921-5093(01)01718-X
- L. Takacs and J. S. McHenry, “Temperature of the Milling Balls in Shaker and Planetary Mills,” Journal of Materials Science, Vol. 41, No. 16, 2006, pp. 5246-5249. doi:10.1007/s10853-006-0312-4
- J. Lee, Q. Zhang and F. Sato, “Mechanochemical Synthesis of LaOX (X=Cl, Br) and Their Solid Solutions,” Journal of Solid State Chemistry, Vol. 160, No. 2, 2001, pp. 469-473. doi:10.1006/jssc.2001.9276
- P. Kovacheva, D. Todorovsky and D. Redev, “Mechanochemistry of the 5f-Elements Compounds. 5. Influence of the Reaction Medium on the Mechanochemically Induced Reduction of U3O8,” Journal of Radioanalytical and Nuclear Chemistry, Vol. 287, No. 1, 2011, pp. 193-197. doi:10.1007/s10967-010-0666-6
- J. Lee, Q. Zhang and F. Sato, “Mechanochemical Synthesis of LaOX (X=Cl, Br) and Their Solid Solutions,” Journal of Solid State Chemistry, Vol. 160, No. 2, 2001, pp. 469-473. doi:10.1006/jssc.2001.9276
- N. Setoudeh, N. J. Welham and S. M. Azami, “Dry Mechanochemical Conversion of SrSO4 to SrCO3,” Journal of Alloys and Compounds, Vol. 492, No. 1-2, 2010, pp. 389-391. doi:10.1016/j.jallcom.2009.11.114
- J. Lee, Q. Zhang and F. Saito, “Synthesis of Nano-Sized Lanthanum Oxyfluoride Powders by Mechanochemical Processing,” Journal of Alloys and Compounds, Vol. 348, No. 1-2, 2008, pp. 214-219. doi:10.1016/S0925-8388(02)00837-X
- S. Bernal, F. J. Botana, R. Garcia and J. M. RodriguezIzquerdo, “Study of the Interaction of Two Hexagonal Neodymium Oxides with Atmospheric CO2 and H2O,” Journal of Materials Science, Vol. 23, No. 4, 1988, pp. 1474-1480. doi:10.1007/BF01154619
- J. Gouteron, D. Michel, A. M. Lejus and J. Zarembowitch, “Raman Spectra of Lanthanide Sesquioxide Single Crystals: Correlation between A and B-Type Structures,” Journal of Solid State Chemistry, Vol. 38, 1981, pp. 288-296. doi:10.1016/0022-4596(81)90058-X
- J. Aride, J.-P. Chaminade and M. Pouchard, “Flux Growth of NdOCl Single Crystals,” Journal of Crystal Growth, Vol. 57, No. 1, 1982, pp. 194-196. doi:10.1016/0022-0248(82)90267-6
- G. D. Del Cul, S. E. Nave, G. M. Begun and J. R. Peterson, “Raman Spectra of Tetragonal Lanthanide Oxychlorides Obtained from Polycrystalline and Single-Crystal Samples,” Journal of Raman Spectroscopy, Vol. 23, No. 5, 1992, pp. 267-272. doi:10.1002/jrs.1250230505
- L.-J. Meng and M. P. dos Santos, “A Study of Residual Stress on rf Reactively Sputtered RuO2 Thin Films,” Thin Solid Film, Vol. 375, No. 1-2, 2000, pp. 29-32. doi:10.1016/S0040-6090(00)01174-3
- L.-J. Meng, V. Teixeira and M. P. dos Santos, “Raman Spectroscopy Analysis of Magnetron Sputtered RuO2 Thin Films,” Thin Solid Films, Vol. 442, No. 1-2, 2003, pp. 93-97. doi:org/10.1016/S0040-6090(03)00953-2
- Y. C. Su, C. A. Chen, Y. M. Chen, Y. S. Huang, K. Y. Lee and K. K. Tiong, “Characterization of RuO2 Nanocrystals Deposited on Carbon Nanotubes by Reactive Sputtering,” Journal of Alloys and Compounds, Vol. 509, No. 5, 2011, pp. 2011-2015. doi:10.1016/j.jallcom.2010.10.121
- The Japan Society of Calorimetry and Thermal Analysis, “Thermodynamic Database MALT for Windows,” ver. 1, Kagaku Gijutsu-Sha, Tokyo, 2004. http://www.kagaku.com/malt/
- H. Samata, D. Kimura, S. Mizusaki, Y. Nagata, T. C. Ozawa and A. Sato, “Synthesis and Characterization of Neodymium Oxyhydroxide Crystals,” Journal of Alloys and Compounds, Vol. 468, No. 1-2, 2009, pp. 566-570. doi:10.1016/j.jallcom.2008.01.056
- M. W. Shafer and R. Roy, “Rare-Earth Polymorphism and Phase Equilibria in Rare-Earth Oxide-Water Systems,” Journal of the American Ceramic Society, Vol. 42, No. 11, 1959, pp. 563-570. doi:10.1111/j.1151-2916.1959.tb13574.x
- J. Cuya, N. Sato, K. Yamamoto, A. Muramatsu, K. Aoki and Y. Toga, “Thermogravimetric Study of the Sulfurization of TiO2 Nanoparticles Using CS2 and the Decomposition of Their Sulfurized Product,” Thermochimica Acta, Vol. 410, No. 1-2, 2004, pp. 27-34. doi:10.1016/S0040-6031(03)00366-6
- A. M. Heyns and K.-J. Range, “Raman and Infrared Study of Neodymium Oxide Peroxide, Nd2O2(O2),” Journal of Raman Spectroscopy, Vol. 25, No. 11, 1994, pp. 855-859. doi:10.1002/jrs.1250251103
- M. Kemdehoundja, J. L. Grosseau, Poussard and J. F. Dinhut, “Raman Microprobe Spectroscopy Measurements of Residual Stress Distribution along Blisters in Cr2O3 Thin Films,” Applied Surface Science, Vol. 256, No. 9, 2010, pp. 2719-2725. doi:10.1016/j.apsusc.2009.11.016
- M. Tanaka, M. Hasegawa, A. F. Dericioglu and Y. Kagawa, “Measurement of Residual Stress in Air PlasmaSprayed Y2O3-ZrO2 Thermal Barrier Coating System Using Micro-Raman Spectroscopy,” Materials Science and Engineering: A, Vol. 419, No. 1-2, 2006, pp. 262-268. doi:10.1016/j.msea.2005.12.034
- I. Ahmad, M. Holtz, N. N. Faleev and H. Temkin, “Dependence of the Stress-Temperature Coefficient on Dislocation Density in Epitaxial GaN Grown on α-Al2O3 and 6H-SiC Substrates,” Journal of Applied Physics, Vol. 95, No. 4, 2004, pp. 1692-1697.doi:10.1063/1.1637707
- H. Kimachi, S. Yamamoto, W. Ota, K. Shirakihara and Y. Fujita, “Measurement of Local Stress Components in Single Crystal Alumina by Using Raman Microspectroscopy with Sub-Micro Spatial Resolution,” Journal of the Society of Materials Science (Japan), Vo. 56, No. 7, 2009, pp. 603-609 (in Japanese). doi:10.2472/jsms.58.603

