Advances in Molecular Imaging
Vol.4 No.3(2014), Article
ID:47607,7
pages
DOI:10.4236/ami.2014.43005
The Role of 18F-FDG-PET/CT in the Management of Patients with High-Risk Breast Cancer: Case Series and Guideline Comparison
Ben F. Bulten1,2, Marie J. de Haas1, Haiko J. Bloemendal3, Adriaan J. van Overbeeke4, Jan Paul Esser1, Henk J. Baarslag5, Lioe-Fee de Geus-Oei2, C. J. Rodenburg3, John M. H. de Klerk1
1Department of Radiology and Nuclear Medicine, Meander Medical Centre, Amersfoort, The Netherlands
2Department of Radiology and Nuclear Medicine, Radboud University Medical Centre, Nijmegen, The Netherlands
3Department of Oncology, Meander Medical Centre, Amersfoort, The Netherlands
4Department of Surgery, Meander Medical Centre, Amersfoort, The Netherlands
5Department of Radiology, Meander Medical Centre, Amersfoort, The Netherlands
Email: ben.bulten@radboudumc.nl, mj.de.haas@meandermc.nl, hj.bloemendal@meandermc.nl, aj.van.overbeeke@meandermc.nl, jp.esser@meandermc.nl, hj.baarslag@meandermc.nl, lioe-fee.degeus-oei@radboudumc.nl, jmh.de.klerk@meandermc.nl
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 24 April 2014; revised 23 May 2014; accepted 23 June 2014
ABSTRACT
Objectives: In grade III-IV breast cancer, dissemination of disease needs to be assessed. Until now this was done by conventional imaging (liver ultrasonography, chest X-ray and bone scintigraphy), but evidence favoring the use of FDG-PET/CT is accumulating. Methods: Patients with high-risk breast cancer, who had received conventional imaging and FDG-PET/CT, were included. Patients were staged and assigned a treatment after 1) conventional imaging and 2) FDG-PET/CT, both by a multidisciplinary oncology team. Equivocal FDG-PET/CT findings were histologically confirmed. Results: 16 patients were included (mean age 59 years). TNM-stage changed in 5 patients (31%) after FDG-PET/CT. In 3 patients (19%) unknown distant metastases were detected by FDG-PET/CT. An adjustment of treatment took place in 4 patients (25%). Conclusions: Our case series emphasizes the role of FDG-PET/CT in the staging of high-risk breast carcinoma, especially in the assessment of distant metastases. We suggest replacing conventional imaging with FDG-PET/CT.
Keywords:Breast Cancer, FDG-PET/CT, Staging, Dissemination, Guidelines

1. Introduction
Annually, 364.000 (or 109 per 100.000) patients are diagnosed with breast cancer in the European Union [1] . Either discovered by mammography screening or through self-examination, in case of a suspicious lesion patients are referred to a radiologist to undergo an ultrasonography with histological confirmation. When this reveals breast cancer, patients are staged according to the TNM-classification and are stratified into a low-risk (stage I and II) and high-risk group (stage III and IV).
In Europe, it is common practice to assess dissemination of disease in grade III-IV breast cancer. Until now, conventional imaging with liver ultrasonography, chest X-ray and bone scintigraphy has been used for this purpose [2] . However, the sensitivity and specificity of these methods are relatively low [2] -[4] , while treatment choice is still based on the results of these tests. Therefore, new modalities with higher sensitivity and specificity in the detection of metastases are needed.
Recently, the Dutch Guidelines for breast carcinoma, developed by NABON, emphasized the relatively new role of (18)-fluorodeoxyglucosis positron emission tomography/computed tomography (FDG-PET/CT) in breast carcinoma staging [5] . After extensive literature study they conclude that patients with a TNM-stage of III are entitled to an FDG-PET/CT because of the better results in detecting metastases. Furthermore, they recommend considering an FDG-PET/CT in stage II primary breast carcinoma and when local recurrent or metastatic disease is suspected. Unfortunately, clinicians still hesitate to implement FDG-PET/CT in the standard care of these patients.
To demonstrate that FDG-PET/CT can change clinical stage and influence treatment decisions, this short patient series was conducted to compare staging and treatment choices based either on conventional imaging or FDG-PET/CT.
2. Materials and Methods
From August 2007 till August 2013, all patients with high risk breast cancer were retrospectively included in the present study when conventional imaging as well as FDG-PET/CT was consecutively performed within 3 months. Conventional imaging consisted of liver ultrasonography, chest X-ray and bone scintigraphy. Liver ultrasonography and chest X-ray were performed routinely. The procedures were assessed by experienced radiologists. The bone scintigram was performed 3 hours after intravenous injection of 500 - 600 MBq of Technetium- 99m-hydroxymethane-diphosphonate. Whole body images from anterior and posterior were obtained simultaneously by a dual-head gamma camera (Symbia T, Siemens Healthcare). The bone scan was assessed by an experienced nuclear medicine physician.
Whole-body PET/CT images were obtained with an integrated 40-MDCT PET/CT scanner (Biograph 40 True Point PET/CT, Siemens Healthcare). Patients fasted for 6 hours before intravenous injection of 3 MBq/kg body weight FDG. Before injection of FDG, blood glucose levels were confirmed to be <11 mmol/L (i.e. <198 mg/dL). Incubation was performed in a warm room with patients in supine position. Sixty minutes after FDG injection, image acquisition was performed. First, low-dose, unenhanced CT images were acquired with the following parameters: 120 kV, 26 - 30 mAs (automatic dose modulation), 0.8-second tube rotation time, pitch of 1.2, and 1.5-mm slice width (reconstructed to contiguous 5-mm axial slices to match section thickness of the PET images). PET-scanning from midfemur to the base of the skull was performed in five or six bed positions, 3 minutes per bed position. Low-dose CT data was used for attenuation correction of the PET images, which were reconstructed with an ordered-subsets expectation maximization algorithm for 14 subsets and four iterations. The image reconstruction matrix was 128 × 128. FDG-PET/CTs were assessed by an experienced nuclear medicine physician.
Disease staging and treatment regimen were formulated by a multidisciplinary oncology team in two sessions based on 1) conventional imaging and 2) FDG-PET/CT. The oncology team consisted of 1 - 2 medical oncologists, an oncologic surgeon, a radiotherapist, a nuclear medicine physician and a radiologist, all with experience in the field of breast carcinoma. When lesions on FDG-/PET-CT were equivocal, histology was obtained to reveal the final diagnosis. Afterwards, staging and treatment adjustments were reviewed by one of the authors (BB).
3. Results
Sixteen patients with high-risk breast carcinoma were included in the study, with a mean age of 59 years (range 33 - 84). The time between conventional and FDG-PET/CT imaging was less than 1 month in 13 cases, 2 months in 2 cases and 3 months in 1 case. The stage of disease after conventional imaging and before FDGPET/CT was IIIA in 4 patients (25%), IIIB in 3 patients (19%), IIIC in 2 patients (13%) and IV in 7 patients (44%). After FDG-PET/CT the stage of disease was IIIA in 4 patients, IIIB in 1 patient (6%), IIIC in 2 patients and IV in 9 patients (56%). In 5/16 patients (31%) TNM-stage changed after FDG-PET/CT: in 4/5 patients this resulted in upstaging of disease, in 1/5 patients in down staging. Examples of these cases are depicted in Figure 1 and Figure 2. In 3 patients (19%) distant metastases were diagnosed after FDG-PET/CT, while conventional imaging did not identify them. Details of TNM-stage changes are described in Table1
After conventional imaging, 8/16 (50%) patients were planned to undergo (neo)-adjuvant chemotherapy and radiotherapy, followed by surgery. 7 patients (44%) were assigned to palliative chemotherapy and 1 to locoregional radiotherapy. After FDG-PET/CT 7/16 patients (44%) were treated with (neo)-adjuvant chemotherapy and radiotherapy with surgery, 8/16 (50%) palliative chemotherapy and 1 locoregional radiotherapy. In 4 patients (25%) a treatment adjustment was induced by FDG-PET/CT results: 2 patients changed from adjuvant chemotherapy and radiotherapy plus surgery to palliative chemotherapy and 1 patient changed vice versa. In 1 patient (no. 3), the field of adjuvant radiotherapy changed due to FDG-PET/CT. All treatment changes took place in patients who had undergone conventional and FDG-PET/CT within 1 month.
4. Discussion
The results of the present study show that the TNM-stage of patients with high risk breast cancer changes in up to 31% when an FDG-PET/CT is used instead of conventional imaging. This resulted in treatment changes in 25% of patients. These changes in treatment are substantial and influence the general management of patients with stage III or IV breast carcinoma.
aCI = conventional imaging: the combination of liver ultrasonography, chest X-ray and bone scintigraphy; bΔ = difference; cAdj. = adjuvant; CT = chemotherapy; RT = radiotherapy; pall. = palliative.
 (a)
(a) (b)
(b) (c)
(c) (d)
(d) (e)
(e) (f)
(f)
Figure 1. Patient no. 2, upgraded to grade IV breast carcinoma after FDG-PET/CT. Chest X-ray (a) and bone scintigraphy (b) without distant metastases. Ultrasonography is not shown here, but did not show metastases. Maximum intensity projection (MIP, (c)) and axial slices of FDG-PET/CT. The axial slices show in detail axillary and mediastinal lymph nodes (d), axillary lymph nodes and pulmonary metastases (arrows) (e) and a bone metastasis (f).
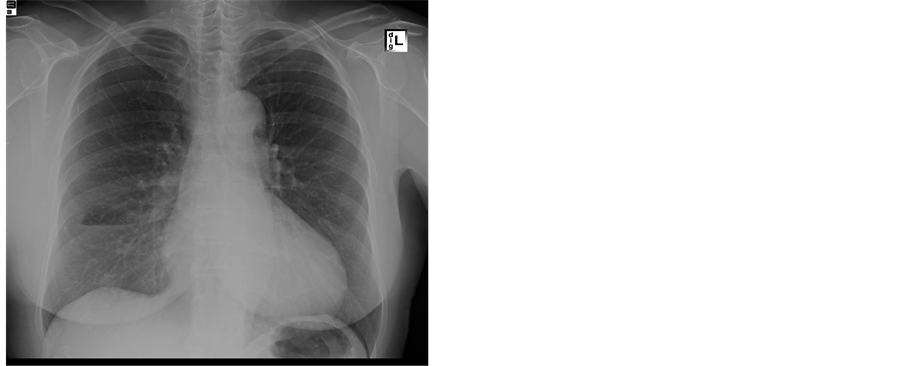 (a)
(a) (b)
(b) (c)
(c) (d)
(d) (e)
(e) (f)
(f) (g)
(g)
Figure 2. Patient no. 14, downgraded to grade III breast carcinoma after FDG-PET/CT. Chest X-ray (a) and bone scintigraphy (b) showed no metastases. Liver ultrasonography (c) showed two lesions suspect for metastases. Axial slices of the CT abdomen ((d) and (e), see arrows) showed the two lesions, metastasis could not be excluded. Axial slices of the FDG-PET/CT ((f) and (g)) did not display increased uptake in those lesions, excluding metastases.
The Dutch guideline for breast cancer recommends the use of FDG-PET/CT in assessing metastatic disease of primary grade III breast carcinoma [5] . This is based on research by several groups [3] [4] [6] -[8] . For example, Fuster et al. conducted a prospective study in 60 patients, showing a sensitivity of 100% and a specificity of 98% for FDG-PET (without CT), compared with a sensitivity of 60% and specificity of 93% in conventional imaging [3] . In another study by Mahner et al. in 199 patients, FDG-PET detected distant metastases with a sensitivity of 87% and a specificity of 86%. This is far better than conventional imaging (43% and 98% respectively), but comparable to CT (83% and 84%) [4] . Koolen et al. recently compared FDG/PET-CT with conventional imaging in 154 patients, resulting in a sensitivity of 100%, specificity of 96%, positive predictive value of 80%, negative predictive value of 100% and an accuracy of 97% [8] .
In all these studies more lesions are detected by FDG-PET/CT than by conventional imaging. This is not always important for clinical issues and decision making. However, change of TNM-classification (but not stage) might have an impact on the clinical setting in terms of prognosis and informing patients. When additional lesions result in a different TNM-stage (e.g. IIIC instead of IV), the impact on management of individual patients is greatly affected. In the present study the change of TNM-stage after FDG-PET/CT resulted in an adjustment of treatment in 25% of patients. Koolen et al. found that in 8% of cases treatment was adjusted after FDG-PET/ CT, but the difference between these numbers can be accounted for by the inclusion of stage III and IV breast carcinoma in our study (and stage II and III in Koolen’s) [8] . Furthermore, one of our patients received a curative instead of a palliative treatment, which can be seen as a downstaging of treatment, while the patients of Koolen were all upstaged. For patients this is, of course, of great importance. Another study in 60 patients with invasive breast cancer larger than 3 cm and/or at least one tumour-positive axillary lymph node, TNM-stage was changed in 17% after FDG-PET/CT and 12% received an alternative radiotherapy approach [2] . Abovementioned studies show that the introduction of FDG-PET/CT results in treatment changes, which is an important clinical finding.
Additionally, the Dutch guideline recommends the use of FDG-PET/CT when local, regional or distant recurrence is suspected [5] . An extensive systematic review by Pennant et al. showed a significantly higher sensitivity and specificity for locoregional recurrence than conventional imaging, as well as a higher sensitivity in comparison with CT [9] . This suggests an improved accuracy in detecting locoregional recurrence when FDG-PET/ CT is added to conventional imaging, also in stage II breast cancer.
Finally, the Dutch guideline supports the use of FDG-PET/CT in equivocal lesions identified by other imaging modalities [5] .
Interestingly, other guidelines are often reticent about the use of FDG-PET/CT in breast carcinoma. For example, the British guideline, published in 2009, advises to use FDG-PET/CT in equivocal lesions, but sees no role for FDG/PET-CT in assessment of disseminated disease. It states that assessment of visceral metastases has to be done by “a combination of plain radiography, ultrasound, CT and MRI” and of bone metastases in “the bony window of the CT or MRI or a bone scintigraphy” [10] . The exact combination or evidence for this advice is lacking. Furthermore, CT might not detect bone metastases at diagnosis, while FDG-uptake is already present, as yet known from lung cancer [11] .
The European Society of Medical Oncology does not recommend the routinely use of FDG-PET/CT as well. Their recently published guideline states that routine staging evaluations are directed at locoregional disease, as asymptomatic distant metastases are very rare and patients do not profit from comprehensive laboratory and radiological staging. They furthermore advise to use FDG-PET/CT only when conventional imaging is inconclusive [12] .
The National Comprehensive Cancer Network (NCCN) also recommends against FDG-PET/CT in their 2009 guideline, because of high false-negative rates in detecting small (<1 cm) lesions, low sensitivity for detecting axillary metastases, low prior probability of the patients having distant metastatic disease and a high rate of false-positive scans [13] . However, modern PET/CT systems have a far better spatial resolution, especially when time-of-flight scanning can be performed, reducing the detection limit of FDG-avid tumors (most breast cancer) to 3 - 4 millimeter. The number of false-negative scans is therefore dramatically reduced. Furthermore, sensitivity for detecting axillary metastases might be low, though still improving, but the specificity in the assessment of axillary lymph nodes is high (96%) [3] . When a positive lymph node is found on FDG-PET/CT, the chance of metastases is high and additional ultrasonography and biopsy need to be done [2] . Above that, non-axillary lymph nodes can be detected by FDG-PET/CT; according to Aukema et al. these are present in 28% of patients with grade II or III breast carcinoma [3] . Finally, when small and non-specific FDG-positive lesions are found (and therefore the chance of false-positivity is high), the Dutch guideline advises to ignore these when a patient can still be cured [2] .
Prior probability of patients having distant disease is relatively low, as posed by NCCN. However, in the present study 19% of patients had distant metastases on FDG-PET/CT that were missed on conventional imaging. These patients did not have to undergo an unnecessary tough and risky therapy regimen with a mastectomy, lymph node dissection, radiotherapy and chemotherapy, which would have been indicated when FDG-PET/CT was not performed.
5. Conclusion
The results of the present study confirm the previously reported impact of FDG-PET/CT in staging and treatment decision making of high-risk breast cancer patients. We suggest to replace conventional imaging with FDG-PET/CT in the assessment of distant metastasis in this category of patients and to adopt this in national and international guidelines, so patients receive the treatment they deserve.
References
- Ferlay, J., Steliarova-Foucher, E., Lortet-Tieulent, J., Rosso, S., Coebergh, J.W., Comber, H., et al. (2013) Cancer Incidence and Mortality Patterns in Europe: Estimates for 40 Countries in 2012. European Journal of Cancer, 49, 1374-1403. http://dx.doi.org/10.1016/j.ejca.2012.12.027
- Aukema, T.S., Rutgers, E.J., Vogel, W.V., Teertstra, H.J., Oldenburg, H.S., Vrancken Peeters, M.T., et al. (2010) The Role of FDG PET/CT in Patients with Locoregional Breast Cancer Recurrence: A Comparison to Conventional Imaging Techniques. European Journal of Surgical Oncology, 36, 387-392. http://dx.doi.org/10.1016/j.ejso.2009.11.009
- Fuster, D., Duch, J., Paredes, P., Velasco, M., Munoz, M., Santamaria, G., et al. (2008) Preoperative Staging of Large Primary Breast Cancer with [18F]fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Compared with Conventional Imaging Procedures. Journal of Clinical Oncology, 26, 4746-4751.http://dx.doi.org/10.1200/JCO.2008.17.1496
- Mahner, S., Schirrmacher, S., Brenner, W., Jenicke, L., Habermann, C.R., Avril, N., et al. (2008) Comparison between Positron Emission Tomography Using 2-[Fluorine-18]fluoro-2-deoxy-D-glucose, Conventional Imaging and Computed Tomography for Staging of Breast Cancer. Annals of Oncology, 19, 1249-1254. http://dx.doi.org/10.1093/annonc/mdn057
- National Breast Cancer Consultation Group (2012) National Guideline Breast Cancer. www.oncoline.nl
- Puglisi, F., Follador, A., Minisini, A.M., Cardellino, G.G., Russo, S., Andreetta, C., et al. (2005) Baseline Staging Tests after a New Diagnosis of Breast Cancer: Further Evidence of Their Limited Indications. Annals of Oncology, 16, 263-266. http://dx.doi.org/10.1093/annonc/mdi063
- van der Hoeven, J.J., Krak, N.C., Hoekstra, O.S., Comans, E.F., Boom, R.P., van Geldere, D., et al. (2004) 18F-2-fluoro-2-deoxy-d-glucose Positron Emission Tomography in Staging of Locally Advanced Breast Cancer. Journal of Clinical Oncology, 22, 1253-1259. http://dx.doi.org/10.1200/JCO.2004.07.058
- Koolen, B.B., Vrancken Peeters, M.J., Aukema, T.S., Vogel, W.V., Oldenburg, H.S., van der Hage, J.A., et al. (2012) 18F-FDG PET/CT as a Staging Procedure in Primary Stage II and III Breast Cancer: Comparison with Conventional Imaging Techniques. Breast Cancer Research and Treatment, 131, 117-126. http://dx.doi.org/10.1007/s10549-011-1767-9
- Pennant, M., Takwoingi, Y., Pennant, L., Davenport, C., Fry-Smith, A., Eisinga, A., et al. (2010) A Systematic Review of Positron Emission Tomography (PET) and Positron Emission Tomography/Computed Tomography (PET/CT) for the Diagnosis of Breast Cancer Recurrence. Health Technology Assessment, 14, 1-103.
- Cancer, N.C.C.F. (2009) Advanced Breast Cancer: Diagnosis and Treatment.
- Fanggiday, J.C., Staaks, G.H.A., de Haas, M.J., Schaefer-Prokop, C.M. and de Klerk, J.M.H. (2011) Discordancy in CT and PET in Detection and Follow Up of Bone Metastases in a Patient with Small Cell Lung Cancer. Dutch Journal of Nuclear Medicine, 33, 558-560.
- Senkus, E., Kyriakides, S., Penault-Llorca, F., Poortmans, P., Thompson, A., Zackrisson, S., et al. (2013) Primary Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Annals of Oncology, 24, vi7-vi23.
- Carlson, R.W., Allred, D.C., Anderson, B.O., Burstein, H.J., Carter, W.B., Edge, S.B., et al. (2009) Breast Cancer. Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network, 7, 122-192.


