Z. ASHRAF ET AL.
260
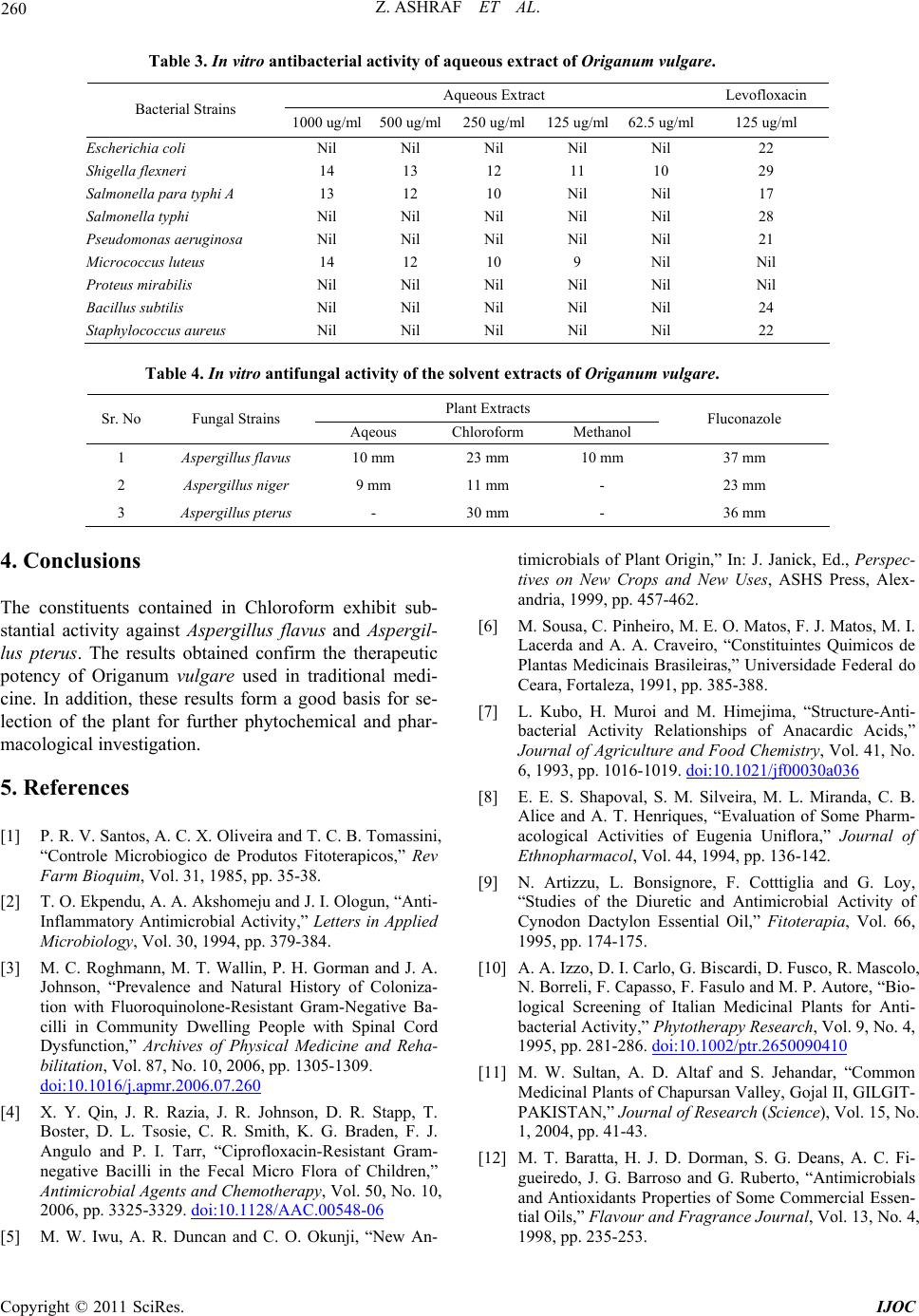
Table 3. In vitro antibacterial activity of aqueous extract of Origanum vulgare.
Aqueous Extract Levofloxacin
Bacterial Strains 1000 ug/ml500 ug/ml250 ug/ml125 ug/ml62.5 ug/ml 125 ug/ml
Escherichia coli Nil Nil Nil Nil Nil 22
Shigella flexneri 14 13 12 11 10 29
Salmonella para typhi A 13 12 10 Nil Nil 17
Salmonella typhi Nil Nil Nil Nil Nil 28
Pseudomonas aeruginosa Nil Nil Nil Nil Nil 21
Micrococcus luteus 14 12 10 9 Nil Nil
Proteus mirabilis Nil Nil Nil Nil Nil Nil
Bacillus subtilis Nil Nil Nil Nil Nil 24
Staphylococcus aureus Nil Nil Nil Nil Nil 22
Table 4. In vitro antifungal activity of the solvent extracts of Origanum vulgare.
Plant Extracts
Sr. No Fungal Strains Aqeous Chloroform Methanol Fluconazole
1 Aspergillus flavus 10 mm 23 mm 10 mm 37 mm
2 Aspergillus niger 9 mm 11 mm - 23 mm
3 Aspergillus pterus - 30 mm - 36 mm
4. Conclusions
The constituents contained in Chloroform exhibit sub-
stantial activity against Aspergillus flavus and Aspergil-
lus pterus. The results obtained confirm the therapeutic
potency of Origanum vulgare used in traditional medi-
cine. In addition, these results form a good basis for se-
lection of the plant for further phytochemical and phar-
macological investig ation.
5. References
[1] P. R. V. Santos, A. C. X. Oliveira and T. C. B. Tomassini,
“Controle Microbiogico de Produtos Fitoterapicos,” Rev
Farm Bioquim, Vol. 31, 1985, pp. 35-38.
[2] T. O. Ekpendu, A. A. Akshomeju and J. I. Ologun, “Anti-
Inflammatory Antimicrobial Activity,” Letters in Applied
Microbiology, Vol. 30, 1994, pp. 379-384.
[3] M. C. Roghmann, M. T. Wallin, P. H. Gorman and J. A.
Johnson, “Prevalence and Natural History of Coloniza-
tion with Fluoroquinolone-Resistant Gram-Negative Ba-
cilli in Community Dwelling People with Spinal Cord
Dysfunction,” Archives of Physical Medicine and Reha-
bilitation, Vol. 87, No. 10, 2006, pp. 1305-1309.
doi:10.1016/j.apmr.2006.07.260
[4] X. Y. Qin, J. R. Razia, J. R. Johnson, D. R. Stapp, T.
Boster, D. L. Tsosie, C. R. Smith, K. G. Braden, F. J.
Angulo and P. I. Tarr, “Ciprofloxacin-Resistant Gram-
negative Bacilli in the Fecal Micro Flora of Children,”
Antimicrobial Agents and Chemotherapy, Vol. 50, No. 10,
2006, pp. 3325-3329. doi:10.1128/AAC.00548-06
[5] M. W. Iwu, A. R. Duncan and C. O. Okunji, “New An-
timicrobials of Plant Origin,” In: J. Janick, Ed., Perspec-
tives on New Crops and New Uses, ASHS Press, Alex-
andria, 1999, pp. 457-462.
[6] M. Sousa, C. Pinheiro, M. E. O. Matos, F. J. Matos, M. I.
Lacerda and A. A. Craveiro, “Constituintes Quimicos de
Plantas Medicinais Brasileiras,” Universidade Federal do
Ceara, Fortaleza, 1991, pp. 385-388.
[7] L. Kubo, H. Muroi and M. Himejima, “Structure-Anti-
bacterial Activity Relationships of Anacardic Acids,”
Journal of Agriculture and Food Chemistry, Vol. 41, No.
6, 1993, pp. 1016-1019. doi:10.1021/jf00030a036
[8] E. E. S. Shapoval, S. M. Silveira, M. L. Miranda, C. B.
Alice and A. T. Henriques, “Evaluation of Some Pharm-
acological Activities of Eugenia Uniflora,” Journal of
Ethnopharmacol, Vol. 44, 1994, pp. 136-142.
[9] N. Artizzu, L. Bonsignore, F. Cotttiglia and G. Loy,
“Studies of the Diuretic and Antimicrobial Activity of
Cynodon Dactylon Essential Oil,” Fitoterapia, Vol. 66,
1995, pp. 174-175.
[10] A. A. Izzo, D. I. Carlo, G. Biscardi, D. Fusco, R. Mascolo,
N. Borreli, F. Capasso, F. Fasulo and M. P. Autore, “Bio-
logical Screening of Italian Medicinal Plants for Anti-
bacterial Activity,” Phytoth erapy Re search, Vol. 9, No. 4,
1995, pp. 281-286. doi:10.1002/ptr.2650090410
[11] M. W. Sultan, A. D. Altaf and S. Jehandar, “Common
Medicinal Plants of Chapursan Valley, Gojal II, GILGIT-
PAKISTAN,” Journal of Research (Science), Vol. 15, No.
1, 2004, pp. 41-43.
[12] M. T. Baratta, H. J. D. Dorman, S. G. Deans, A. C. Fi-
gueiredo, J. G. Barroso and G. Ruberto, “Antimicrobials
and Antioxidants Properties of Some Commercial Essen-
tial Oils,” Flavour and Fragrance Journal, Vol. 13, No. 4,
1998, pp. 235-253.
Copyright © 2011 SciRes. IJOC