Agricultural Sciences
Vol.3 No.3(2012), Article ID:19045,11 pages DOI:10.4236/as.2012.33052
Effect of salinity and drought on symbiotical and biochemical properties of Onobrychis and alfalfa
![]()
1Institute of Microbiology, Uzbek Academy of Sciences, Tashkent, Uzbekistan; *Corresponding Author: zair@dostlink.net
2Scientific Center of Plant Production “Botanika”, Uzbek Academy of Sciences, Tashkent, Uzbekistan
Received 19 January 2012; revised 24 February 2012; accepted 9 March 2012
Keywords: Onobrychis Species; Alfalfa; Nodule Bacteria; Nodule; Salinity; Drought; Enzyme Isoforms
ABSTRACT
To search for an alternative to alfalfa under conditions of salinity and drought, a comparative study was carried out to explore the effect of salinity on the symbiosis of alfalfa and local esparcet species (Onobrychis transcaucasica and Onobrychis chorassanica) inoculated with their nodule bacteria. The salinity of up to 30 mM NaCl insignificantly affected the biomass growth of shoots and roots of alfalfa plants, but the increase in the salinity from 30 to as high as 140 mM NaCl led to the biomass decrease. The salinity produced a double effect on the nodulation process in inoculated alfalfa plants as follows: 1) at 30 - 100 mM NaCl the stimulation of nodulation and increased leghemoglobin activity were observed; 2) at salinity concentrations higher than 100 mM NaCl the suppression of both nodule formation and leghemoglobin activity was observed. Alfalfa plants under inoculation with the Sinorhizobium meliloti 10 strain obtained a consider-able resistance to salinity (50 - 80 mM NaCl). The efficient symbiosis of O. transcaucasica plants with Rhizobium sp. OT111 and O. chorassanica plants with Rhizobium sp. OC109 enhanced the adaptation of plants to salinity up to 150 mM NaCl. The gradual growth suppression of both Onobrychis plants species started from 200 mM NaCl, and salinity concentration 300 mM NaCl was critical (sub-lethal) for plants independently of inoculation by nodule bacteria. In field conditions, O. chorassanica was more resistant to salinity than O. transcaucasica, but minimal irrigation for both species of Onobrychis showed a higher effect on their growth and development than the moderate salinity at the concentration 75 mM NaCl. The lower limit (drought threshold) of drought-resistance of Onobrychis plants was 6% - 8% of soil humidity. In shoot and roots of alfalfa, both Onobrychis plant species subject to salt stress, aldehyde oxidase and xanthine dehydrogenase enzymes and different number of their isoforms as well as their electrophoretic mobilities/activities were found.
1. INTRODUCTION
Legume grasses are used in agriculture under crop rotation for restoration of biological productivity of soils and their enrichment by biological nitrogen through the symbiosis of grasses together with nitrogen-fixing nodule bacteria. Alfalfa is used widely in many countries for this purpose and it is sowed in the overall are about of 32 million ha. The cultivation of it is conducted preferably on well-irrigated non-saline soils. However, due to the global warming and deficit of water resources in some regions (Central Asia, Kazakhstan) the area of desert and salt-affected soils increases, which threatens stable harvests in agriculture. Since the zone of fertile lands is shrinking the sowing area for alfalfa also shrinks because of a lower productivity of this agricultural crop in soils subject to such stresses as salinity and drought. Esparcet, Onobrychis, is a perennial leguminous grass that is widely distributed in many natural and climatic areas, some species are used in agriculture of many countries [1-3]. Onobrychis chorassanica grows in adyrs (non-irrigated steppe massifs) in Central Asia, but in sub-mountain and plain regions of Central Asia Onobrychis transcaucasica species was introduced before; it can be characterized as a leguminous grass that favorably develops under valley conditions without any need in special irrigation [4].
Stresses (salinity and drought) damage the functioning of many biological processes in living organisms, their growth and development including nitrogen assimilation by plants as well as the biological nitrogen fixation with nitrogen-fixing microorganisms [5]. The intake of assimilable nitrogen forms into living organisms declines, but the addition of mineral nitrogen forms from outside can partially compensate nitrogen metabolism losses caused by stresses.
Aldehyde oxidase (AO; EC 1.2.3.1) and xanthine dehydrogenase (XDH; EC 1.2.1.37) are known to take part in processes connected with the adaptation of living organisms to stress conditions. So, for example, АО (aldehyde oxidase, ЕС 1.2.3.1) catalyzes the last stages in biosynthesis of two phytohormones—oxidation of abscisic aldehyde up to abscisic acid and oxidation of indole-3-acetaldehyde up to indole-3-acetic acid [6-8]. XDH takes part in a purine metabolism and also in biosynthesis of ureides in higher plants; ureides like urea, as “scavengers”, could remove oxygen radicals, which are formed under stress conditions [9,10].
The aim of the present study was to obtain a comparative evaluation of salinity and drought effect on symbiotic properties (growth, development and nodulation) of both alfalfa “Tashkent-1728” plant species sowed in Uzbekistan and inoculated by nodule bacteria, and two local Onobrychis perennial leguminous grass species, Onobrychis chorassanica and Onobrychis transcaucasica, not requiring irrigation, as well as on biochemical properties (AO and XDH activities) of plants and their nodule bacteria.
2. MATERIALS AND METHODS
2.1. Determination Salt Resistance of Alfalfa and Onobrychis Plants
The seeds of alfalfa “Tashkent-1728” variety, O. transcaucasica and O. chorassanica were treated with concentrated sulphuric acid for 4 min, followed by repeated washings with sterile water. The treated seeds were germinated under sterile conditions on wet filter paper discs placed in Petri dishes, for 2 days at 30˚C. The germinated seeds were planted in 2 L bags (two seeds/ bag) filled with sand impregnated with the following basal nutrient medium [11]: MgSO4·4H2O—5 mM, K2SO4—10 mM, CaCl2·2H2O—1 mM, phosphates buffer—15 mM (NaH2PO4 + Na2HPO4, pH 6.5), Fe-Sequestrene 138 (Fe-EDDHA)—60 mg/L and 0.05 ml/L of a microelements solution containing (g/L): H3BO3—17.16, MnSO4— 7.2, ZnSO4—1.32, CuSO4—1.65 and Na2MoO4—0.12. After appearance of seedlings of germinated seeds, they were inoculated with bacterial suspensions of 3-daily nodule bacteria cultures that were prepared in the nutritive medium solution in titer 109 cells/ml (on 2 ml of microelements solution per each tube together with 10 ml of bacterial suspension in the nutritive medium per each bag). The plants seedlings were inoculated with nodule bacteria Sinorhizobium meliloti 8, 10, 71; Rhizobium sp. OT111, OT115, OT117 (nodule bacteria of O. transcaucasica); Rhizobium sp. ОС107, OC109, OC138 (nodule bacteria of O. chorassanica). The following treatments plants were set up: nutrient medium without nitrogen and nutrient medium containing 1 mM NH4NO3. Each of these treatments was carried out in the absence of salinity and in the presence from 10 to 200 mM NaCl for the alfalfa and from 50 to 400 mM NaCl for the plants Onobrychis. Variants of inoculation were done in 6 repeats on 2 plants per each repeat. Every 5 days the bags were watered with 0.5 L of nutrient solution so as to allow for the equilibration of the potting material to the desired salinity level. Plants were grown under natural conditions in the garden in summer time. After two month, plants were harvested, separated into roots and shoots and dried for 24 h at 75˚C.
Microplot trial tests on salt-resistance of Onobrychis plants were conducted in serozem soils plots of 210 m2. Area of each variant was 7 m × 10 m = 70 m2. There were 30 rows for every variant (15 rows for the O. transcaucasica and on 15 rows for the O. chorassanica). Length of row was 7 m and width of row was 0.3 m. The seeds of plants were sowed into soil by burring to the depth of 3 cm. Inoculation of seeds before sowing was done by bacterial suspension of Rhizobium sp. OС109 with titre 109 cells/ml. Varians of treatment included following conditions: control—every week plants watering with 10 m3 fresh water; minimal irrigation—every week plants watering with 5 m3 fresh water; salinity— two week plants watering with 10 m3 salt water (80 mM NaCl) and two week watering with 10 m3 fresh water. Plans were grown up during 3 months (May-July).
2.2. Drought-Resistance Study of Onobrychis Plants
The experiment on drought-resistance of Onobrychis symbiosis was carried out in field conditions during 3 months. Soil humidity was measured with help of TDRmethod and gravimetric method (weighing of soil samples). Soil was taken from 0 - 30 сm horizons and twice was dried up at 105˚C; the difference between mass initially-taken and mass of dried up samples was determined which was evaluated as soil humidity. Plants seeds inoculated with Rhizobium sp. OC109 were sown in microplot trial test bed. The distance between plants was 25 cm (length of bed—2.5 m, width of bed—0.25 m). The first soil watering was conducted for equalizing of soil moisture at experimental plot for better growth of Onobrychis seedlings. After week the soil humidity under from plants was about 20%. Next watering of adjoining soil (not including the square occupied by planted plants) was conducted from one side of beds with planted plants. In whole, there were 3 such watering (irrigations, 1 irrigation per month) during all period of plants growing up in order to obtain a gradient of soil moisture. The gradient of soil moisture was obtained due to that irrigation was done on the one hand (side of the first plants within bed) towards opposite side of the last plants within bed where there were dried up soils containing lesser water. As a result, the gradient of soil moisture under the plants at the microplot was obtained.
2.3. Obtaining of Enzyme Extracts from Plant and Nodule Bacteria
To obtain enzyme extracts the different parts of alfalfa plants (nodules and roots) grown during 40 days at different NaCl concentrations, leaves and roots of Onobrychis plants grown during 15 days in salinity conditions were used. Plant materials were introduced into mortar, where 250 mM Tris-HCl (pH 8.5) was added, 1 mM EDTA, 1 mM DTT, 5 mM L-Cys, 80 mM Na2MoO4, 0.1 mM PMSF, 10 mM GSH, and 0.03 mM FAD and glass sand in ratio 1:3:1 were added too, i.e. 1 part of plant biomass, 3 parts of buffer and 1 part of glass sand. The mortar content was cooled up to 4˚C and grinded up to homogenous state. The homogenate was centrifuged at 13,000g during 30 min at 4˚C. Rhizobium sp. OT111 and Rhizobium sp. OC109 nodule bacteria were grown for 2 days on medium of the following composition (g/L): glucose 5, sucrose—5, K2НРО4—0.5, KН2РО4—0.5, MgSO4∙7H2O—0.5, CaSO4—0.2, pea—50, water distilled—up to 1L, pH 6.8 - 7.0 (pea was boiled during 1 hour and the medium was prepared on the basis of pea’s broth). At the 3rd day NaCl was introduced into cultural suspension up to final concentration 1 M and the bacteria cultivation was continued during the day. Nodule bacteria biomass was collected by centrifugation at 6000 g during 30 min. Bacteria biomass was resuspended in above-mentioned buffer and cells of culture were disintegrated with help of ultrasonic during 1.5 min at 4˚C. The ratio bacteria biomass and buffer was 1:2. Bacteria homogenate was centrifugated at 13,000 g for 30 min at 4˚C. Supernatants of plants and bacteria served as a source of enzyme extract for study of aldehyde oxidase and xanthine dehydrogenase enzymes.
2.4. Aldehyde Oxidase and Xanthine Dehydrogenase Assay
AO activity was detected in polyacrylamide gels by staining after native electrophoresis with 7.0% acrylamide gels [12] in the absence of SDS at 4˚C. The gel was immersed in a reaction mixture containing 0.2 M phosphate buffer, pH 7.5, 0.1 M Tris-HCl (pH 7.5), 0.1 mM phenazine methosulfate (PMS), 1 mM 3[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium-bromide (MTT) and 1 mM substrate indole-3-aldehyde for 10 min followed by gentle shaking at room temperature.
XDH activity was detected after native gel-electrophoresis using hypoxanthine as a substrate [13], which resulted in the development of specific formazan bands.
3. RESULTS
3.1. Salt-Resistance of Alfalfa and Onobrychis Species Plants in Dependence on Growth Conditions
Vegetation experiments showed that low (up to 30 mM) NaCl concentrations insignificantly influenced on growth biomass increment of shoot and root parts in comparison with variants without salinity treatment in the presence 1 mM NH4NO3 (+N) and in the absence (–N) of exogenous nitrogen. The further increase of salt concentration (from 30 to 140 mM NaCl) led to biomass lowering (Table 1). Plant biomass in variants inoculated with S. meliloti No.8 nodule bacteria in the presence of exogenous nitrogen (+N) at 30 mM NaCl concentration by 1.4 times exceeded (–N) plant biomass. In spite of good growth and plant development in the presence of exogenous nitrogen the increase of plant tolerance to salinity was not observed. Alfalfa plants in symbiosis with S. meliloti No.10 nodule bacteria acquired a considerable resistance to salinity, i.e. at 50 - 80 mМ NaCl salinity the lowering of plant biomass came only to 11% - 19% in comparison with control. If in the value of ratio “shoot biomass”/“root biomass” in control comprised 2 and more, then under salinity it gradually lowered in dependence on salinity concentration. Nodules had pink colour up to 80 - 100 mM NaCl and white-pink colour at salt concentrations from 100 to 140 mM NaCl. The critical (baleful) NaCl concentration for alfalfa plants survival was 200 mM NaCl, while the concentrations from 80 to 100 mM NaCl influenced negatively already on growth and plant development. “Nodule-forming capacity” of salt-resistant rhizobia for alfalfa plants remained up to 140 mM NaCl. The increase of NaCl concentrations from 30 to 100 mM in variants without addition of exogenous nitrogen didn’t essentially influence on nodules formation in plant roots. With increase salt concentrations up to 140 mM NaCl in all variants of treatment the suppression of growth and alfalfa plants development was observed.
Study of Onobrychis plants salt-resistance in variants inoculated with Rhizobium sp. OT111 and Rhizobium sp. OT117 nodule bacteria strains showed that at salinity concentration 50 - 100 mM NaCl the lowering of shoot biomass of O. transcaucasica plants was not observed practically comparing to the plants not subjected to salinity stress (Table 2). The further increase of salinity up to 150 mM NaCl led to biomass lowering by only 7.6% -
Table 1. Influence of salinity on growth, development and nodulation of “Tashkent-1728” alfalfa plants symbiosis (vegetation experiments).
9.1%. O. transcaucasica plants in symbiosis with Rhizobium sp. OT115 strain didn’t give positive results in salnity conditions. But the negative influence of salinity on growth and plant development was prevented by addition of 1 mM NH4·NO3, exogenous nitrogen, i.e. in consequence of assimilation of bound nitrogen the plants got a considerable resistance to salinity comparingly to control plants inoculated with only Rhizobium sp. OT115 nodule bacteria.
Analogous results were obtained also for O. сhorassanica plants (Table 3). In salinity conditions (50 - 100 mM NaCl) O. chorassanica plant biomass increased by 22.5% - 64% in the presence of exogenous nitrogen. When inoculation of O. chorassanica plants with Rhizobium sp. OC107 and Rhizobium sp. OC109 strains the resistance of plants to salinity stress increased 150 mM NaCl and plant biomass loss came to 1.8% - 4.1% in comparison with control. The further increase of NaCl concentration led to lowering of plant biomass (both shoot and root parts) of both Onobrychis species. It should be noted that the ratio of the shoot part to the root part of Onobrychis plants was comparatively the same, i.e. the ratio of biomass of shoot part to root part was equal to 1. Salinity 300 mM NaCl was critical (sublethal) for Onobrychis plants independently on either their inoculation by nodule bacteria or the presence of bound nitrogen in plant growth media.
If salinity value 140 mM NaCl was actually lethal for


Table 2. Influence of salinity on growth, development and nodulation of Onobrychis transcaucasica plants symbiosis (vegetation experiments).
alfalfa (salinity threshold for alfalfa—50% biomass loss— came to 100 mM NaCl), then at salinity 200 - 300 mM and even at 400 mM NaCl the nodules were still formed on Onobrychis roots, salinity threshold (50% biomass loss) NaCl and enhancement of adaptation to salnity stress depended on symbiotic efficiency of nodule bacteria or exogenous nitrogen addition in stage of plants development. Onobrychis plants two-fold exceeded indices of salt-resistance for alfalfa on both shoot part biomass and root part biomass that certified in favor that Onobrychis plants really are the best alternate for alfalfa in salinity conditions.
Thus, salinity exerted the double effect on nodule formation process of alfalfa inoculated plants, 1) within the range from 30 to100 mM NaCl concentrations the stimulation of nodulation (in comparison with control)


Table 3. Influence of salinity on growth, development and nodulation of Onobrychis chorassanica plants symbiosis (vegetation experiments).
and increased leghemoglobin activity were observed, 2) at concentrations higher than 100 mM NaCl the suppression of both nodule formation and leghemoglobin activeity was observed. In case of Onobrychis plants such critical effect—salinity threshold—was within the range 200 mM NaCl. The presence of added nitrogen, on the one hand, supported the growth and plant development, but on the other hand, it lowered the nodule formation at subcritical and critical salinity concentrations. Virulence (nodulating activity) of salt-tolerant rhizobia for alfalfa plants was found even at 140 mM NaCl concentration, but for Onobrychis plants—up to 300 mM NaCl. Salinity exercises more harmful influence on macrosymbiont (host plant—alfalfa, Onobrychis) than on microsymbiont (nodule bacteria), that at moderate and higher salt concentrations the bacteria still keep their virulence (nodulating ability). As a result of salt-tolerance study of Onobrychis plants it was revealed that significant increase of plant adaptation to salinity (150 mM NaCl) is supported at the expense of efficient symbiosis O. transcaucasica plants together with Rhizobium sp. OT111, OT117 nodule bacteria and O. chorassanica plants—Rhizobium sp. OC107, OC109 nodule bacteria.
In order to clear up which of stresses—salinity or drought—exerts the maximal influence on both Onobrychis species the comparative study of these stresses acting in moderate (subcritical) dose on inoculated plants of both Onobrychis species was undertaken. The comparative study of stress action—moderate salinity (in concentration 75 mM NaCl) or moderate drought (minimal irrigation)—on growth and plant development of both O. transcaucasica and O. chorassanica in field conditions showed that minimal irrigation of Onobrychis plants influenced on growth and development of the plants more negatively than salinity did. In all variants the plant seedlings germinated by 3 - 4th day after sowing of plant seeds, during the first 15 days plants developed equally independently on type and character of stress.
The gradual suppression of plant growth was observed in 1-monthly plants of O. transcaucasica and O. chorassanica in variants of minimal irrigation and under salinity (75 mM NaCl) in relation to control. By the second month of growing up the plants bloomed and even in O. chorassanica plants the seed formation began. Under salinity and minimal irrigation the shoot part biomass of O. transcaucasica plants came to 82% and 75% accordingly from control plant biomass (Figure 1). O. chorassanica plant biomass under salinity reduced insignificantly 5% (Figure 1), but at minimal irrigation –45%.
3.2. Drought Resistance of Onobrychis Plants
Besides revelation of salt-resistance threshold equally important was to determine the limit of drought resistance for Onobrychis plants. For this purpose it was undertaken a study of growth of inoculated Onobrychis plants on soil moisture content (Figure 2). As result it
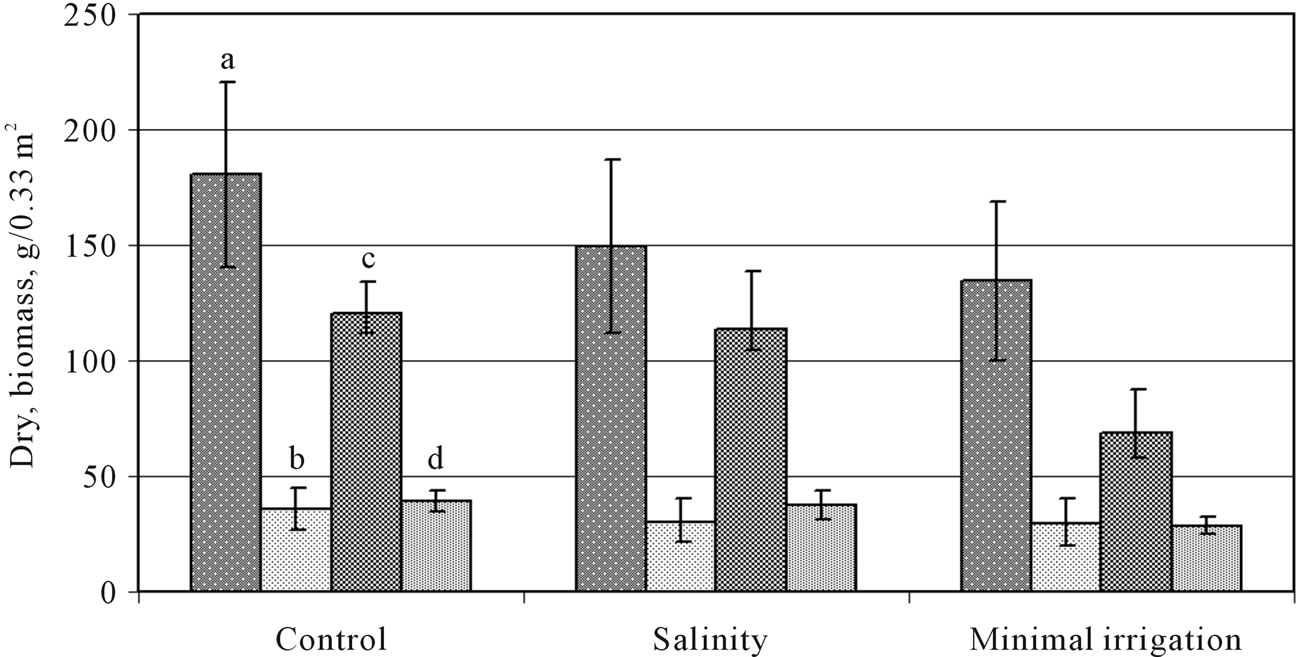
Figure 1. Influence of stress factors on biomass of shoot (a) and root (b) O. transcaucasica plants, shoot (с) and root (d) O. chorassanica plants in field conditions.
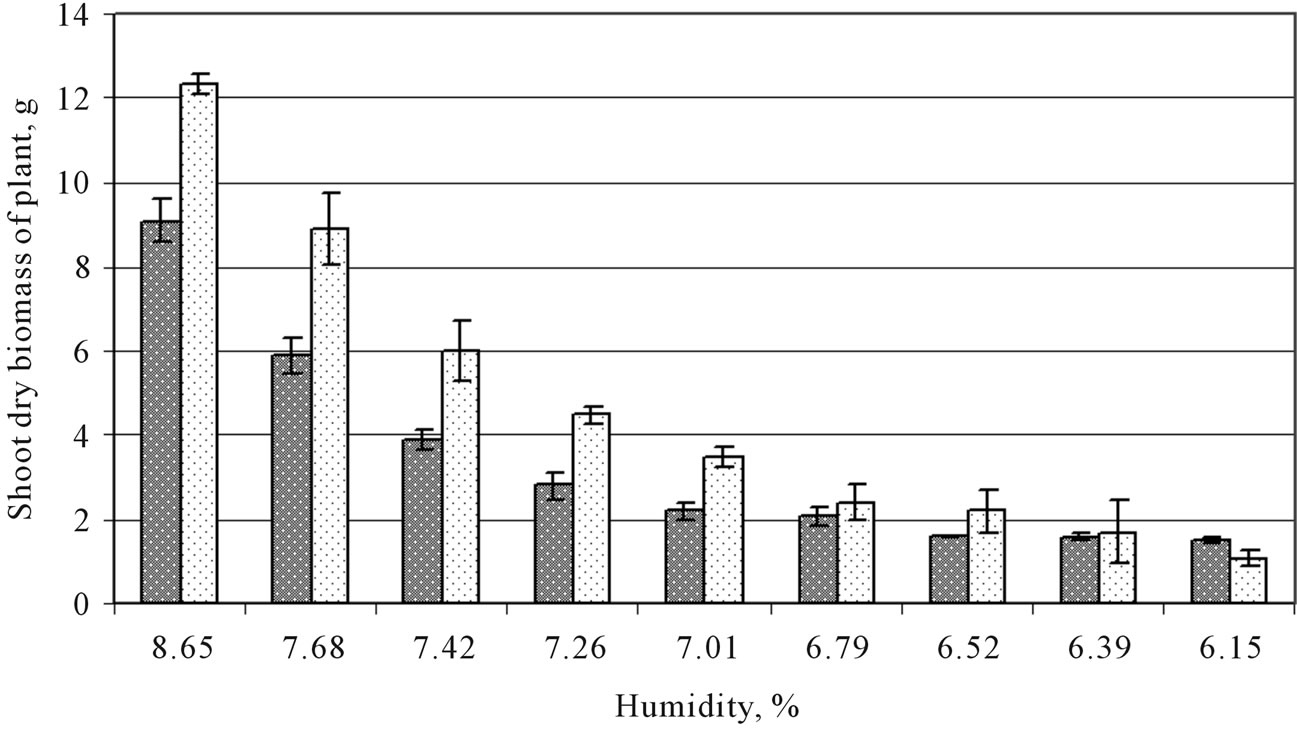
Figure 2. Dependence of growth and plant development of O. transcaucasica (a) and O. chorassanica (b) plants on soil moisture.
has been obtained a gradient of soil moisture under the plants within range from 8.65% to 6.15%. In course of growing up it was detected a gradient of plants biomass— from the side of conducted watering to the contrary (opposite) side, but decrease of plant height gradient (O. transcaucasica and O. chorassanica) was not such sharp as it took place in case of plant biomass gradient. Under this the biomass of inoculated plants for both Onobrychis species with decrease of soil moisture also decreased regularly. The maximal shoot biomass of inoculated O. transcaucasica plants was 9.1 g/1 plant and for O. chorassanica—12.3 g/1 plant (under 8.65% of moisture), under 6.15% in case of O. transcaucasica the biomass decreased more than 6 times (1.49 g/1 plant), when biomass of O. chorassanica—more than in 11 times (1.1 g/1 plant). At the same time the indices of plants height were as follows: under 8.65% of moisture 33.3 cm (for O. transcaucasica) and 38.0 cm (for O. chorassanica), whereas under 6.15% of moisture these indices were 22.0 cm and 21.6 cm correspondingly (Figure 2). On the basis of conducted experiments it may conclude that insignificant change of soil moisture led to significant changes of plant biomass and lesser changes—for plant height. In these experiments even the difference in 2.5% of soil moisture (between the maximal and minimal observed soil moisture indices) determined more favorable conditions for obtaining higher biomass harvest of Onobrychis plants.
Thus, Onobrychis perennial leguminous grass has much greater potential of resistance to stresses (salinity and drought) than alfalfa and it may be used for recultivation and restoration of salt-affected and deserted lands as alternative of alfalfa in crop rotation of ecologicallyunfavourable soils.
3.3. Aldehyde Oxidase and Xanthine Dehydrogenase of Alfalfa and Onobrychis Plants and Their Nodule Bacteria in Salnity Conditions
The next task was a study of aldehyde oxidase (AO) and xanthine dehydrogenase (XDH) activities in different parts (shoot, root, nodules) of salt-affected alfalfa and Onobrychis plants as well as to study the same activities of their nodule bacteria. It is known that АО and XDH take part in processes, connected with adaptation of living organisms to stress conditions, in particular, in biosynthesis of two phytohormones (abscisic acid and indole-3-acetic acid), participation in purine metabolism and ureide biosynthesis that remove oxygen radicals which are formed in stress conditions.
The availability of AO and XDH isoforms was studied by electrophoresis method in polyacrylamide gel (PAAG) with following zymograms based on enzyme activity. In our experiments when studying AOand XDH-activities in different parts of alfalfa plants (shoot, root and nodules) subjected to salt stress the availability of one AO form in shoot and root of plants was found. Two AO forms were found in nodules (plant part)—AO1 and AO2, at that AO2 had high electrophoretic mobility and level of activity (Figure 3). Such big difference on electrophoretic mobility between AO2 and AO1 pointed at

Figure 3. AO and XDH activities in different parts of alfalfa “Tashkent-1728” (shoot, root and nodule plant part) grown in different salinity range of NaCl.
that AO2 molecular weight could be much less than analogous one of AO1. It should be noted that with increase of NaCl concentration in growth medium the intensity of AO activity from different parts of plant organs increased as a natural result in response to salt stress. Before some authors noted the increased AO activity and availability of 3 AO isoforms on pea leaves in salinity conditions and nutrition of plants with ammonium [14]. Other authors also observed analogous picture and availability of 4 AO-isoforms in maize (Zea mays L.) under salinity, but it should be noted that in both works the electrophoretical mobilities of AO-isoforms were very similar [15]. Study of XDH-isoforms (originated from different alfalfa organs) showed the availability of one XDH-form with low activity (Figure 3) in shoot and root of alfalfa, while 3 XDH forms were revealed in nodules which had similar electrophoretic mobilities, XDH2 and XDH3 had low activity in comparison with XDH1 form. With increase of salinity (closer to 80 - 120 mM NaCl) the activities of XDH2 and XDH3 increased (the color intensity of zymograms increased). The search of analogs that was conducted by us to study of AO and XDH enzymes in nodule plant part of legume plants showed that such works were absent, while other authors were studying extensively superoxide dismutase (SOD), peroxidase (POD), ascorbate peroxidase (APX), and catalase (CAT), in alfalfa shoots and roots subjected to salt and drought stresses during germination [16]. Thus, nodule is the most sensitive and operational organ in legume plants, the adaptation in which allows them to occupy wide ranging areas in stress-subjected conditions in environment. In experiments in both Onobrychis species (in root and shoot samples) the availability of AO has been determined. In both O. transcaucasica control and salt-treated (150 mM NaCl) samples, grown under correspondent conditions, one AO form has been found in shoot and root samples (Figure 4). In roots of O. chorassanica, subjected to the salt stress, the availability of two AO forms has been found. It should be noted that AO1 activity was very weak in relation to AO2 activity (Figure 4).
At the same time, in shoot part of O. chorassanica the availability of two AO (AO1, AO2) forms has been found both in control and salt-affected samples. As the study of XDH activity in different parts of O. transcaucasica showed, only one XDH form has been found (Figure 4). In shoot part of O. chorassanica two XDH forms with different activities have been determined (Figure 4). The XDH form (originated from root) and XDH1 form (originated from shoot) of O. chorassanica had similar electrophoretic mobilities.
The study of availability of aldehyde oxidase in Rhizobium sp. OT111 and Rhizobium sp. OC109 bacteria did not give positive results, i.e. in tested nodule bacteria cultures the AO enzyme has not been found. From Figure 4 it is seen, that Rhizobium sp. OT111 and Rhizobium sp. OC109 bacteria cultures had one definite XDH form and the enzyme activity intensity depended on growth conditions. It has been established that XDH activity of nodule bacteria was significantly higher under
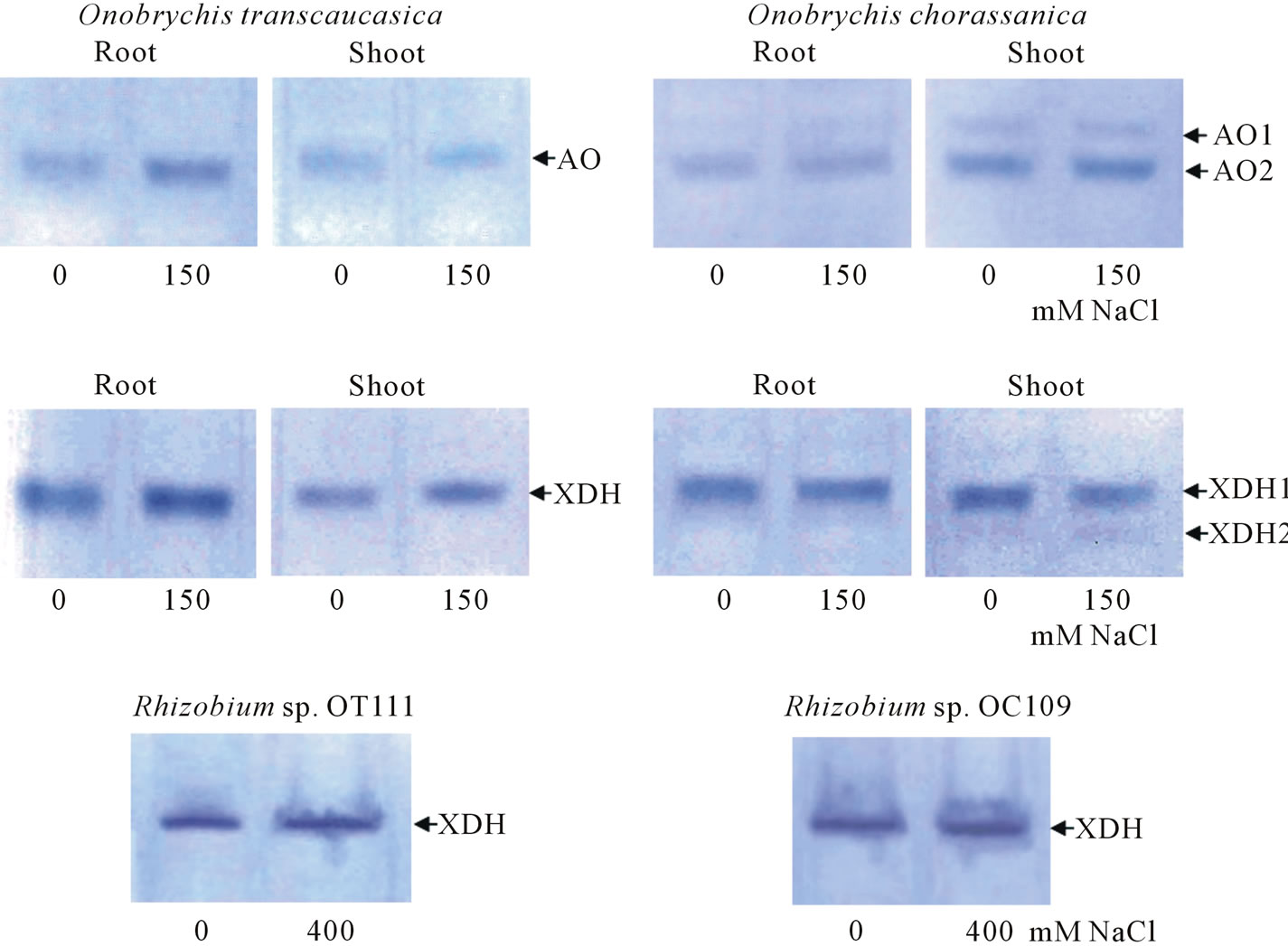
Figure 4. AO and XDH activities in root, shoot parts of O. transcaucasica, O. chorassanica and their nodule bacteria grown in different salinity.
their growth in medium containing 400 mM NaCl than analogous one in control variants.
Thus, one may conclude that AO and XDH activity levels and their isoform availability in shoot and root parts of O. transcaucasica and O. chorassanica plants as well as in both of nodule bacteria depended on growth conditions, displayed more isoforms (shoot part of O. chorassanica) and their intensity (activity) increased during salinity treatment. Since AO enzyme is absent in nodule bacteria and AO-activity (color intensity in zymograms) in Onobrychis plants subjected to salinity was low, then probably it makes sense with help of genetic engineering methods it might to introduce the multicopy of AO-gene into genome of nodule bacteria and use nodule bacteria as a vector system for synthesis of isoforms of this enzyme within symbiotic system in order to increase the adaptation of Onobrychis nitrogen-fixing symbiosis to stresses exposure.
4. DISCUSSION
If in irrigated agricultural lands alfalfa produces a good yield of green biomass under favorable conditions (without stresses) and its nitrogen-fixing symbiosis together with nodule bacteria is a very efficient measure to increase biological productivity and soil fertility (up to 100 - 200 kg of biological nitrogen per ha), then in saltaffected and drought-subjected soils it has low yield of green biomass and correspondingly its contribution into soil fertility restoration is small. Plants of both Onobrychis species grow in natural habitats owing to rainfall; O. chorassanica grows in steppe ecosystem subject to drought and salinity and, in this connection, Onobrychis shows a higher potential for adaptation to stresses than alfalfa and, correspondingly, more chances to survive in ecologically-unfavorable lands. The enhancement of adaptation of investigated plants to salinity was achieved by inoculation of plants with efficient nodule bacteria, in particular, salt resistance of alfalfa plants in symbiosis with S. meliloti 10 strain increased up to 50 mM NaCl, symbiosis of O. transcaucasica plants with Rhizobium sp. OT111 strain and O. chorassanica plants with Rhizobium sp. OC107, OC109 strains increased the adaptation of plants to salinity up to 150 mM NaCl. Perhaps during the efficient symbiosis the osmoprotectants are synthesized and accumulated intensively at the expense of nitrogen fixation in response to an increased osmosis from the external environment [17]. The experiments on minimal irrigation and salinity treatment showed that Onobrychis plants need more irrigation and with a minimal sufficient irrigation they could be the best alternative of alfalfa in conditions of droughtand salt-affected soils, since the salt resistance threshold for Onobrychis exceeds threefold the analogous value for alfalfa.
Both macrosymbiont (legume host plant), synthesizing of enzyme isoforms responsible for adaptation, and microsymbiont (nodule bacteria), synthesizing of leghemoglobin and keeping ability to form nodules on host plant under stress, take part in process of adaptation to stresses. Salinity showed a more negative effect on macrosymbiont than on microsymbiont that may be noted in the change of color intensity of enzyme isoform activity, which are responsible for adaptation under the effect of increased salt concentrations.
REFERENCES
- Rumball, W. (1982) Plant introduction trials. Performance of sainfoin (Onobrychis viciifolia Scop.) and related species at Palmerston North. New Zealand Journal of Experimental Agriculture, 10, 383-385.
- Laguerre, G., Van Berkum, P., Amarger, N. and Prévost, D. (1997) Genetic diversity of rhizobial symbionts isolated from legume species within the genera Astragalus, Oxytropis, and Onobrychis. Applied Environmental Microbiology, 63, 4748-4758.
- Suriyagoda, L.D., Ryan, M.H., Renton, M. and Lambers H. (2010) Multiple adaptive responses of Australian native perennial legumes with pasture potential to grow in phosphorusand moisture-limited environments. Annals of Botany, 105, 755-767. doi:10.1093/aob/mcq040
- Khasanov, O.K., Tadjiev, S.F., Shamsuvalieva, L., Konycheva, V.I. and Rakhimova, T. (1983) Onobrychis chorassanica Bge.—Esparcette chorasanskii. In: Saidov, D.K., Ed., Adaptation of Forage Plants to Conditions of Arid Zone of Uzbekistan, “Fan” of Uzbek SSR, Tashkent, 236- 245.
- Elboutahiri, N., Thami-Alam, I. and Udupa, S.M. (2010) Phenotypic and genetic diversity in Sinorhizobium meliloti and S. medicae from drought and salt affected regions of Morocco. BMC Microbiology, 20, 10-15.
- Walker-Simmons, M., Kudra, D.A. and Warner, R.L. (1989) Reduced accumulation of ABA during water stress in a molybdenum cofactor mutant of barley. Plant Physiology, 90, 728-733. doi:10.1104/pp.90.2.728
- Leydecker, M.T., Moureaux, T., Kraepiel, Y., Schnorr, K. and Caboche, M. (1995) Molybdenum cofactor mutants, specifically impaired in xanthine dehydrogenase activity and abscisic acid biosynthesis, simultaneously overexpress nitrate reductase. Plant Physiology, 107, 1427- 1431.
- Koshiba, T., Saito, E., Ono, N., Yamamoto, N. and Sato, M. (1996) Purification and properties of flavinand molybdenum-containing aldehyde oxidase from coleoptiles of maize. Plant Physiology, 110, 781-789.
- Nguyen, J. (1986) Plant xanthine dehydrogenase: Its distribution, properties and function. Physiologie Vegetale, 24, 163-281.
- Triplett, E.W., Blevins, D.G. and Randall, D.D. (1980) Allantoic acid synthesis in soybean root nodule cytosol via xanthine dehydrogenase. Plant Physiology, 65, 1203- 1206. doi:10.1104/pp.65.6.1203
- Hoagland, D.R. and Arnon, D.J. (1938) The water culture method for growing plants without soil. California Agricultural Experimental Station, 347, 1-32.
- Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680-685. doi:10.1038/227680a0
- Mendel, R.R. and Muller, A. (1976) A common genetic determination of xanthine dehydrogenase and NR in N. tabacum. Biochemie und Physiologie der Pflanzen, 170, 538-541.
- Zdunek, E. and Lips, S.H. (2001) Transport and accumulation rates of abscisic acid and aldehyde oxidase activity in Pisum sativum L. in response to suboptimal growth conditions. Journal of Experimental Botany, 52, 1269- 1276. doi:10.1093/jexbot/52.359.1269
- Barabas, N.K., Omarov, R.T., Erdei, L. and Lips, S.H. (2000) Distribution of the mo-enzymes aldehyde oxidase, xanthine dehydrogenase and nitrate reductase in maize (Zea mays L.) nodal roots as affected by nitrogen and salinity. Plant Science, 155, 49-58. doi:10.1016/S0168-9452(00)00199-0
- Wang, W.B., Kim, Y.H., Lee, H.S., Kim, K.Y., Deng, X.P. and Kwak, S.S. (2009) Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiology and Biochemistry, 47, 570-577. doi:10.1016/j.plaphy.2009.02.009
- Verdoy, D., Coba De La Pena, T., Redondo, F.J., Lucas, M.M. and Pueyo, J.J. (2006) Transgenic Medicago truncatula plants that accumulate proline display nitrogen- fixing activity with enhanced tolerance to osmotic stress. Plant, Cell and Environment, 29, 1913-1923. doi:10.1111/j.1365-3040.2006.01567.x

