International Journal of Clinical Medicine
Vol.4 No.1(2013), Article ID:27353,6 pages DOI:10.4236/ijcm.2013.41011
Successful Treatment of Life-Threatening Cerebral Bleeding Associated with Disseminated Intravascular Coagulation Using Recombinant Human Soluble Thrombomodulin in a Patient with Mixed Phenotype Acute Leukemia with t (9; 22) (q34; q11.2); Bcr-Abl1
![]()
1Department of Hematology, National Hospital Organization Disaster Medical Center, Tachikawa, Tokyo, Japan; 2Department of Medical Informatics, National Hospital Organization Disaster Medical Center, Tachikawa, Tokyo, Japan; 3Department of Hematology, National Center for Global Health and Medicine, Tokyo, Japan.
Email: *ntakezak@tdmc.hosp.go.jp
Received November 27th, 2012; revised December 28th, 2012; accepted January 17th, 2013
Keywords: Mixed Phenotype Acute Leukemia with t (9; 22) (q34; q11.2); Bcr-Abl1; Recombinant Human Soluble Thrombomodulin; Imatinib; Disseminated Intravascular Coagulation
ABSTRACT
Recently, mixed phenotype acute leukemia (MPAL) with t (9; 22) (q34; q11.2); bcr-abl1 was described as one kind of acute leukemia of ambiguous lineage in the 2008 World Health Organization Classification of Tumors of Hematopoietic and Lymphoid Tissues. However, treatment strategy remains difficult for this uncommon MPAL. In addition, this type of MPAL is at high risk of tumor lysis syndrome (TLS) because of high chemo-sensitivity. Here, we report a MPAL with t (9; 22) (q34; q11.2); bcr-abl1 case that suffered from life-threatening cerebral bleeding associated with disseminated intravascular coagulation (DIC) with TLS after bcr-abl positive acute lymphoblastic leukemia (ALL) type induction therapy who was successfully treated with recombinant human thrombomodulin (rhTM). This case reached complete remission without additive cerebral bleeding. In conclusion, bcr-abl positive ALL type induction therapy was effective for MPAL with t (9; 22) (q34; q11.2); bcr-abl1 and rhTM was effective against DIC with TLS.
1. Introduction
Mixed phenotype acute leukemia (MPAL) with t (9; 22) (q34; q11.2); bcr-abl1 was described as one kind of acute leukemia of ambiguous lineage in the 2008 World Health Organization (WHO) Classification of Tumors of Hematopoietic and Lymphoid Tissues [1]. Sometimes, patients are found to have high WBC counts of more than 1.00 × 1011/L [2]. In addition, this very rare leukemia is sensitive to chemotherapy. Therefore, it is considered that this type of MPAL is at high risk of tumor lysis syndrome (TLS) [3,4]. If TLS happens, rapid release of procoagulant materials, or enzymes from blasts, or the fibrinolytic activity of leukemic cells themselves is thought to cause disseminated intravascular coagulation (DIC) [5].
DIC is a lethal complication because it is followed by multiple organ failure (MOF) or bleeding [6]. Conventional treatment of DIC has been administration of heaprin. Other agents such as antithrombin (AT) and activated protein C (APC) have been used [7,8]. However, these are not better alternatives which have clear efficacy over heparin in the treatment of DIC. Furthermore, there is reluctance to use these agents in patients of severe bleeding. Recombinant human soluble thrombomodulin (rhTM) has been developed as a novel agent against DIC and has been examined in a randomized clinical trial in Japan [9,10]. The efficacy of this agent over heparin is that the resolution of DIC was significantly better in the group treated with rhTM than in the group treated with unfractionated heparin (UFH) and the incidence of bleeding-related adverse reactions was significantly lower in that with rhTM than in that with UFH [10].
This report shows a patient with mixed phenotype acute leukemia with t (9; 22) (q34; q11.2); bcr-abl1 who suffered life-threating cerebral hemorrhage due to DIC but was treated successfully with rhTM.
2. Case Report
A 49-year-old man with a month-term cough and slight fever was admitted to our hospital on November 4, 2011. Hematological examination revealed that hemoglobin was 8.8 g/dL, white blood cell count was 406,100/µL, blast cell count was 361,400/µL, and platelet count was 3.0 × 104/µL. Other results of the coagulation test were as follows: prothrombin time (13.7 seconds; normal range, 10.5 - 13.5 seconds), activated partial thromboplastin time (aPTT) (25.5 seconds; normal range, 25 - 45 seconds), fibrinogen (393 mg/dL; normal range, 200 - 400 mg/dL), FDP (55.0 µg/mL; normal range, <5 µg/mL), antithrombin III (87.2%; normal range, 80% - 130%), thrombin-antithrombin III complex (TAT) (≧60 µg/L; normal range, 1.0 - 4.1 µg/L), and plasmin-α2-plasmin inhibitor complex (PIC) (18.2 µg/mL; normal range <0.8 µg/L). Results of renal function tests showed the following abnormalities: blood urea nitrogen (33.4 mg/dL; normal range, 8.0 - 22.0 mg/dL), serum creatinine (3.20 mg/dL; normal range, 0.60 - 1.10 mg/dL), estimated glomerular filtration rate (18.48 mL/min/1.73 m2), and uric acid (12.3 mg/dL: normal range, 3.6 - 8.0 mg/dL). Examination of bone marrow aspirate revealed hypercellular marrow with 93.8% peroxidase negative blasts, which were positive for CD13, CD33, CD34, CD10, CD19, and HLA-DR (Figures 1(a), (b), and (c)). These findings suggested mixed phenotype acute leukemia according to the WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. The patient was treated with acute lymphoblastic leukemia (ALL) induction protocol: cyclophosphamide (CPA), daunorubicin (DNR), vincristine (VCR), and prednisolone (PSL). Chromosome analysis showed complex abnormalities, including +6, +8, and +der (22), in addition to t (9; 22) (q34; q11.2) (i.e., Philadelphia chromosome) (Figure 2 and Table 1). Fluorescence in situ hybridization (FISH) analysis for the BCR/ABL fusion gene revealed 100 (cells/100 cell). Therefore additional clinical and laboratory findings subsequently confirmed the diagnosis of MPAL with t (9; 22) (q34; q11.2); bcr-abl1. The patient was treated with imatinib when the Philadelphia chromosome was shown 7 days after the start of induction therapy. Clinical course is shown in Figure 3 and laboratory data is shown in Table 2.
After administration of induction therapy, the patient’s blast cell count decreased to 145,200/µL. However, the patient showed a loss of consciousness on the second day of induction treatment. Urgent examination with computed tomography (CT) showed cerebral hemorrhage and
 (a)
(a) (b)
(b) (c)
(c)
Figure 1. Bone Marrow findings; (a) May-Grunwald-Giemsa—stained bone marrow smears showing a mixed-cell population of large and small blasts. (b) Blasts were negative with myeloperoxidase staining. (c) FACS analysis reveals that blasts were positive CD13, CD33, CD34, CD10, CD19, and HLA-DR. Furthermore, blasts were negative CD1, CD2, CD3, CD4, CD7, CD8, CD14, CD20, CD41, CD56, and GP-A.
 (a)
(a) (b)
(b) (c)
(c)
Figure 2. Chromosome analysis shows complex abnormalities; (a) 46, XY, t (9; 22) (q34; q11.2) 16/20 cells; (b) 47, idem, +der (22) t (9; 22) 3/20 cells; (c) 49, idem, +6, +8, +der (22) t (9; 22) 1/20 cells.

Figure 3. Clinical course of the patient, Abbreviations: Induction: induction therapy (cyclophosphamide, daunorubicin, vincristine, and prednisolone); Recomodulin®: recombinant human thrombomodulin; PC: platelet concentrate; FFP: fresh frozen plasma; Fib: fibrinogen; FDP: fibrin/ fibrinogen degradation products; PT: prothrombin time; TAT: thrombin-antithrombin III complex; PIC: plasmin-α2 -plasmin inhibitor complex.
Table 1. Results of chromosome analysis.

Table 2. Summary of the laboratory results before and after treatment; Abbreviation; Plt: platelet; INR: international normalized ratio; PT: prothrombin time; Fib: fibrinogen; FDP: fibrin/fibrinogen degradation products.

infarction (Figure 4(a)). On the same day, hematological studies showed the following severe coagulation abnormalities: platelet count 25,000/µL; fibrinogen, 26 mg/dL; prothrombin time, 20.2 seconds; aPTT was impossible to count; FDP, 157.7 µg/mL; TAT, >60.0 µg/L, and PIC, 18.2 µg/mL. Diagnosis of DIC was made on the patient’s admission according to the diagnostic criteria of
 (a)
(a)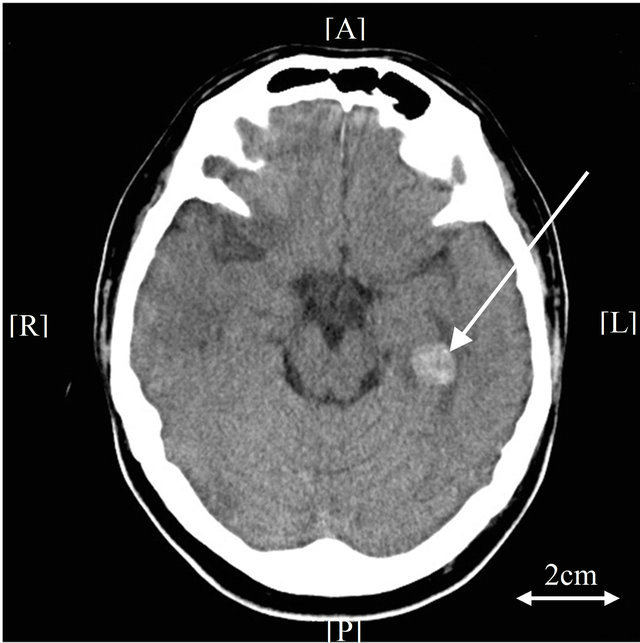 (b)
(b) (c)
(c)
Figure 4. Brain computed tomography (CT), (a) Brain CT shows cerebral hemorrhage and infarction (day 2). The arrow indicates hemorrhage; (b) Follow-up brain CT exam shows slight exacerbation of hemorrhage (day 8). The arrow indicates hemorrhage; (c) No prognosis is observed on further follow-up brain CT exam (day 13). The arrow indicates the improvement of hemorrhage.
the International Society of Thrombosis and Hemostasis [11]. Although the patient had already been treated for DIC with rhTM at the time of his cerebral hemorrhage, we continued to use rhTM as an agent for DIC control (rhTM, 180 IUkg per day). Even though on the 4th day of induction therapy, FDP levels increased temporarily, they deceased from the 5th day. Increased fibrinogen levels over 100 mg/dL were already observed on the third day of induction therapy. TAT and PIC levels were 7.1 µg/L and 5.2 µg/mL on the 14th day of induction therapy. Consciousness was gradually recovered from the 4th day of induction. Although follow-up CT examination 6 days after the first exam showed slight exacerbation of hemorrhage, no prognosis was observed on day 11 (Figures 4(b), (c)). Examination of bone marrow aspirate revealed the rate of blasts was 0.4%, and FISH showed a rate of fusion signals of 5% on the 35th day of induction therapy. The patient achieved complete remission.
3. Discussion
MPAL with t (9; 22) (q34; q11.2); bcr-abl1is a very rare type of acute leukemia [1,2,12]. MPAL cases have been classified as biphenotypic acute leukemia (BAL) on the basis of the EGIL scoring system [13]. The WHO classification has established and published new criteria for the diagnosis of BAL. It has also adopted a new designation for this disease, now termed MPAL [1]. Among them, MPAL with t (9; 22) (q34; q11.2); bcr-abl1 is categorized when blasts have either the t (9; 22) (q34; q11.2) translocation detected by classical karyotyping, or when the bcr-abl gene is detected by FISH or polymerase chain reaction. Sometimes, patients are found to have high WBC counts of more than 1.00 × 1011/L [2]. Sensitivity to induction therapy is good; however, the relapse rate is very high [2,14,15]. 1-year estimated overall survival rate was very low, even if patients achieved complete remission. There are no agreed treatment protocols for patients with MPAL with t (9; 22) (q34; q11.2); bcr-abl1. Some investigators reported that induction treatment with combined acute myeloblastic leukemia (AML)/ALL drugs led to a high rate of early deaths, so it was recommend that induction therapy should be with either AML or ALL drugs followed by stem cell transplantation for younger patients [2,14,15]. In addition, the impact of administration of imatinib has also been reported [2,14,15]. Furthermore, it was reported that there was no apparent difference in survival between patients with bcr-abl positive MPAL and matched patients with bcr-abl positive ALL [14]. Our patient was also treated with ALL type monotherapy combined with imatinib as induction therapy and achieved CR, similar to the literature. These facts may indicate that bcr-abl positive ALL type therapy followed by stem cell transplantation would be standard therapy in the future for this very rare MPAL. Further study is warranted.
TLS is can be a life-threatening complication during induction chemotherapy in patients with acute leukemia [3,4]. Risk factors of TLS were defined as high WBC count, high uric acid, high LDH, and high creatinine levels. In many cases, the rapid release of procoagulant materials or enzymes from blasts or the fibrinolytic activity of leukemic cells themselves is thought to cause DIC [5]. Conventional therapy for DIC with TLC in hematologycal malignancy used heparin or serine protease inhibitor (SPI) as an anticoagulant agent. However, it is difficult to use heparin for DIC with bleeding because of worsening of complications and SPI because of not being enough to control DIC. Thrombomodulin (TM) is an endothelial anticoagulant factor, which is a kind of thrombin receptor that forms a 1:1 complex with thrombin. In this complex, thrombin activates protein C to produce activated protein C (APC), which inactivates factors Va and VIIIa. Thus, TM converts thrombin from a procoagulant protease to an anticoagulant [16]. A phase 3 randomized study demonstrated that the DIC resolution rate in an rhTMtreated group (65.6%) was significantly higher than that in a heparin-treated group (45.9%). In the rhTM-treated group, the number of patients which were not able to continue to be administrated rhTM was two of 116 patients, because of bleeding related adverse reactions, while in the heparin-treated group; this number was 7 of 115 patients [10,17]. TM also has a unique amino-terminal structure exhibiting anti-inflammatory activity. TM binds to high-mobility group box 1 (HMGB1) and helps to digest HMGB1 by thrombin. HMGB1 was identified as a lethal late-phase mediator and is suspected to be correlated with the development of DIC during sepsis [18,19]. Our patient was found to have high WBC counts, high LDH, high uric acid, and high creatinine levels. Therefore, we performed generous intravenous hydration and oral allopurinol before induction chemotherapy according to recommendations [3]. However, this case suffered life-threatening cerebral bleeding because of severe DIC with TLS. We considered the severity of DIC with TLS; therefore, we decided to treat DIC with rhTM and transfusion of coagulation factors (i.e. transfusion of fresh frozen plasma). Our patient achieved CR without additive bleeding. rhTM is not only an anti-coagulation drug but also has an anti-inflammatory effect via the HGMB1 path-way.
In conclusion, bcr-abl positive ALL type induction therapy was effective for MPAL with t (9; 22) (q34; q11.2); bcr-abl1 and rhTM was a very powerful weapon against DIC with TLS. A prospective study should be done to establish treatment strategies for this very rare MPAL.
REFERENCES
- S. H. Swerdlow, E. Campo, N. L. Harris, et al., “Acute Leukemias of Ambiguous Lineage,” In: S. H. Swerdlow, E. Campo, N. L. Harris, et al., Eds., World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues, IARC Press, Lyon, 2008, pp. 150-155.
- Y. Wang, M. Gu, Y. Mi, L. Qiu, S. Bian and J. Wang, “Clinical Characteristics and Outcomes of Mixed Phenotype Acute Leukemia with Philadelphia Chromosome Positive and/or Bcr-Abl Positive in Adult,” International Journal of Hematology, Vol. 94, No. 5, 2011, pp. 552- 555. doi:10.1007/s12185-011-0953-1
- P. Tosi, G. Barosi, C. Lazzaro, V. Liso, M. Marchetti, E. Morra, A. Pession, G. Rosti, A. Santoro, P. L. Zinzani and S. Tura, “Consensus Conference on the Management of Tumor Lysis Syndrome,” Haematologica, Vol. 93, No. 12, 2008, pp. 1877-1885. doi:10.3324/haematol.13290
- N. Zojer and H. Ludwig, “Hematological Emergencies,” Annals of Oncology, Vol. 18, No. Supplement 1, 2007, pp. i45-i48. doi:10.1093/annonc/mdl450
- H. Wada, T. Nagano, M. Tomeoku, M. Kuto, Y. Karitani, K. Deguchi and S. Shirakawa, “Coagulant and Fibrinolytic Activities in the Leukemic Cell Lysates,” Thrombosis Research, Vol. 30, No. 4, 1983, pp. 315-322. doi:10.1016/0049-3848(83)90223-2
- M. Levi and H. T. Cate, “Disseminated Intravascular Coagulation,” The New England Journal of Medicine, Vol. 341, No. 8, 1999, pp. 586-592. doi:10.1056/NEJM199908193410807
- T. Iba, E. Nakarai, T. Takayama, K. Nakajima, T. Sasaoka and Y. Ohno, “Combination Effect of Antithrombin and Recombinant Human Soluble Thrombomodulin in a Lipopolysaccharide Induced Rat Sepsis Model,” Critical Care, Vol. 13, No. 6, 2009, p. R203. doi:10.1186/cc8210
- K. Yamakawa, S. Fujimi, T. Mohri, H. Matsuda, Y. Nakamori, T. Hirose, O. Tasaki, H. Ogura, Y. Kuwagata, T. Hamasaki and T. Shimazu, “Treatment Effects of Recombinant Human Soluble Thrombomodulin in Patients with Severe Sepsis: A Historical Control Study,” Critical Care, Vol. 15, No. 3, 2011, p. R123. doi:10.1186/cc10228
- S. Moll, C. Lindley, S. Pescatore, D. Morrison, K. Tsuruta, M. Mohri, M. Serada, M. Sata, H. Shimizu, K. Yamada and G. C. White, “Phase I Study of a Novel Recombinant Human Soluble Thrombomodulin, ART-123,” Journal of Thrombosis and Haemostasis, Vol. 2, No. 10, 2004, pp. 1745-1751. doi:10.1111/j.1538-7836.2004.00927.x
- H. Saito, S. Maruyama, S. Shimazaki, Y. Yamamoto, N. Akikawa, R. Ohno, A. Hirayama, T. Matsuda, H. Asakura, M. Nakashima and N. Aoki, “Efficacy and Safety of Recombinant Human Soluble Thrombomodulin (ART-123) in Disseminated Intravascular Coagulation: Results of a Phase III, Randomized, Double-Blind Clinical Trial,” Journal of Thrombosis and Haemostasis, Vol. 5, No. 1, 2007, pp. 31-41. doi:10.1111/j.1538-7836.2006.02267.x
- F. B. Taylor Jr, C. H. Toh, W. K. Hoots, H. Wada and M. Levi, “Towards Definition, Clinical and Laboratory Criteria and a Scoring System for Disseminated Intravascular Coagulation,” Thrombosis and Haemostasis, Vol. 86, No. 5, 2001, pp. 1327-1330.
- E. Matutes, W. F. Pickl, M. Van’t Veer, et al., “MixedPhenotype Acute Leukemia: Clinical and Laboratory Features and Outcome in 100 Patients Defined According to the WHO 2008 Classification,” Blood, Vol. 117, No. 11, 2011, pp. 3163-3171. doi:10.1182/blood-2010-10-314682
- M. C. Bene, G. Castoldi, W. Knapp, et al., “Proposals for the Immunological Classification of Acute Leukemias. European Group for the Immunological Characterization of Leukemias (EGIL),” Leukemia, Vol. 9, No. 10, 1995, pp. 1783-1786.
- S. Killick, E. Matutes, R. L. Powles, et al., “Outcome of Biphenotypic Acute Leukemia,” Haematologica, Vol. 84, No. 8, 1999, pp. 699-706.
- Y, Zhang, D, Wu, A. Sun, et al., “Clinical Characteristics, Biological Profile, and Outcome of Biphenotypic Acute Leukemia: A Case Series,” Acta Haematologica, Vol. 125, No. 4, 2011, pp. 210-218. doi:10.1159/000322594
- I. Maruyama, “Recombinant Thrombomodulin and Activated Protein C in the Treatment of Disseminated Intravascular Coagulation,” Thrombosis and Haemostasis, Vol. 82, No. 2, 1999, pp. 718-721.
- E. Ogawa, H. Yagasaki, M. Kato, et al., “Successful Treatment of Disseminated Intravascular Coagulation in a Child with Acute Myelogenous Leukaemia Using Recombinant Thrombomodulin,” British Journal of Haematology, Vol. 149, No. 6, 2010, pp. 911-912. doi:10.1111/j.1365-2141.2010.08135.x
- K. Abeyama, D. M. Stern, Y. Ito, et al., “The N-Terminal Domain of Thrombomodulin Sequesters High-Mobility Group-B1 Protein, a Novel Anti-Inflammatory Mechanism,” The Journal of Clinical Investigation, Vol. 115, No. 5, 2005, pp. 1267-1274.
- T. Ito, K. Kawahara, K. Okamoto, et al., “Proteolytic Cleavage of High Mobility Group Box 1 Protein by Thrombin-Thrombomodulin Complexes,” Arteriosclerosis, Thrombosis, and Vascular Biology, Vol. 28, No. 10, 2008, pp. 1825-1830. doi:10.1161/ATVBAHA.107.150631
NOTES
*Corresponding author.

