Advances in Chemical Engineering and Science
Vol.07 No.02(2017), Article ID:75827,7 pages
10.4236/aces.2017.72017
Synthesis of Crystallized BaWO4 Nanorods in a Microemulsion System
Jie Zhang1,2, Xiaoshu Zhu1,2, Heyong Huang1,2, Yinping Zhang1,2*
1Nanjing Normal University Center for Analysis and Testing, Nanjing, China
2Biomedical Materials Testing Service Center in Jiangsu Province, Nanjing, China

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: February 24, 2017; Accepted: April 25, 2017; Published: April 30, 2017
ABSTRACT
BaWO4 nanorods have been successfully synthesized in w/o microemulsion system containing barium ions via a simple reaction between Ba2+ and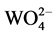 . The BaWO4 Nanorods were characterized by XRD, TEM, and SEM, respectively. Results showed that the solvents composition―volume ratio of 4-dio- xane and distilled water―played the key role in the formation of BaWO4 Nanorods. Furthermore, the strong vibration at 925 cm−1 on its Raman spectrum indicated that the BaWO4 nanorods is good at stimulating Raman scattering in transient and steady-state, making it as a promising candidate material for laser with self-raman conversion of radiation inside the active medium.
. The BaWO4 Nanorods were characterized by XRD, TEM, and SEM, respectively. Results showed that the solvents composition―volume ratio of 4-dio- xane and distilled water―played the key role in the formation of BaWO4 Nanorods. Furthermore, the strong vibration at 925 cm−1 on its Raman spectrum indicated that the BaWO4 nanorods is good at stimulating Raman scattering in transient and steady-state, making it as a promising candidate material for laser with self-raman conversion of radiation inside the active medium.
Keywords:
BaWO4 Nanorods, Microemulsion, 4-Dioxane, Raman Spectrum

1. Introduction
Nowadays, tungstate materials, BaSO4, have attracted much attention in view of its luminescent behavior and structural properties [1] [2] . As compared to other materials, the narrow line width of its stimulated Raman scattering (SRS)-active mode in BaSO4 crystal (1.6 cm−1) leads to high peak intensity (63%). In parti- cular, Raman gain has been measured to be 8.5 cm/GW at 1.06 μm wavelength [2] . Furthermore, the material is not hydroscopic and transparent in visible and near-infrared spectral range. It is a promising material for crystalline nano- and picoseconds Raman lasers. A number of methods, including hydrothermal method, flux method and solid-state reaction [3] - [8] , have been developed to generate tungstate materials. However, tough reaction conditions, such as high- reaction temperature, long-reaction time or complex equipment, were applied in most this approaches [9] . Thus, seeking efficient but low-cost techniques for synthesizing BaSO4 is required for the development of electro-optical materials.
It is well known that different surfactants can form micelles with variable morphologies. This can be utilized for the modification of crystal growth [10] [11] [12] . For example, Zhang et al. [12] reported that the penniform super structures of BaWO4 nanowires have been successfully synthesized in reverse micelles by using a block copolymer as the directing agent. The effects of the mixing ratio between the anionic and cationic surfactants on the crystal growth of BaWO4 nanowires have been further studied. As proposed, the different morphologies and sizes of BaWO4 crystals could be synthesized by the employ- ment of super-molecule templates composed of biomembrane and organic reagents at room temperature [13] . However, the BaWO4 nanoparticles with high crystalline and various regular shapes are required in order to enhance physiccal properties. It has thus been indicated that the BaWO4 microparticles with well crystallinity should be obtained through simple method. Whereas, the composition of the microemulsion influences the structures of the surfactant aggregation as well as the size and shape of the final nanocrystals [14] [15] .
Therefore, the present study was conceived to develop BaWO4 nanoparticles in a water-in-oil (W/O) microemulsion system composed of 1,4-dioxane and water. The phases, morphologies, and luminescent properties have been investigated.
2. Experimental Section
BaCl2∙2H2O and Na2WO4∙2H2O with analytical grade purity were used as, and all other chemical reagents were analytical grade. BaWO4 nanoparticles were obtained from BaCl2 and Na2WO4 microemulsion system according to the following reaction:
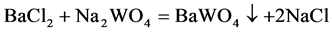
Firstly, 4-dioxane and distilled water were mixed together to prepare a microemulsion system with different volume ratios. Secondly, BaCl2∙2H2O (0.061 g) and Na2WO4∙2H2O (0.0821 g) were dissolved in an aliquot of 10 mL of microemulsion system, respectively. The mixture containing Na2WO4 were then added dropwise into the flask containing BaCl2 at 35˚C and 150 rpm. After mixing for 10 min, the sample was centrifuged at 4024 ×g for 5 min and the white precipitates were collected. Finally, the white precipitates were rinsed three times with distilled water and dried at 80˚C for 6 h.
The X-ray diffraction (XRD) patterns were recorded using a Janpan Ridaku D/Max-γA X-ray diffractometer. The particles size and morphology were cha- racterized by scanning electron microscopy (SEM, JEM-200CX) and transmission electron microscopy (TEM, JEOL-2010). Raman spectra were measured at the 514.5 nm line of an Ar laser (Labram HR800).
3. Results and Discussion
The obtained BaWO4 particle in water-in-oil (W/O) microemulsion system was characterized by XRD, and the typical XRD pattern was shown in Figure 1, in which all the peaks could be indexed to the pure BaWO4 particle with a tetragonal unit cell (a = b = 0.5626 nm, c = 1.2744 nm). This can be indexed to the JCPDS Card No. 82,457 [12] . No other peaks were detected in the pattern, indicating the high purity of the BaWO4 particle.
The typical TEM images of products synthesized with different ratio of 4- dioxane and distilled water were displayed in Figure 2. Figure 2(a) showed that BaWO4 particles with large side length were developed without the addition of 4-dioxane.Whereas, as can be seen from Figure 2(b) and Figure 2(c), with an increase of the ratio of 4-dioxane and distilled water from 3:7 to 5:5, the diame- ter of produced rods significantly reduced from 500 nm to 30 nm. Finally, as indicated from Figure 2(d), when the ratio value reached 6:4, it totally transformed from rods to fine spherical particles. These images implied that the concentration of 4-dioxane played a crucial role in the formation of BaWO4 nanorods. Generally, the formation of BaWO4 crystal basically consists of a nucleation step followed by particle growth stages: In the initial stage, some of nuclei were formed. However, in the particle growth stages, the development of the crystallite is controlled by 4-dioxane. 4-dioxane is also considered as a stabilizer preventing the aggregation during the formation of nanocrystals. Thus, no special morphologies would form in the single aqueous system in the absence of 4-dioxane because no formation of templates for the preparation of BaWO4 nanorods. It is well known that different concentrations of surfactants can form micelles with varied morphologies [15] , which could be utilized for the modifi- cation of crystal growth. Nevertheless, higher concentration of 4-dioxane on BaWO4 crystals resulted in an isotropic growth mode and nearly equiaxial particles, which unfavored the formation of BaWO4 nanorods. Similar results have also been reported by other groups [14] [16] [17] . Additionally, the TEM and
Figure 1. XRD patterns of BaWO4 Nanorods.


Figure 2. TEM images of the produced BaWO4 with different ratio of 1,4-dioxane and water. (a) 0:100; (b) 2:8; (c) 5:5; (d) 6:4.
SEM image of BaWO4 nanorods prepared in the solvents with the ration value of 4:6, was showed in Figure 3. The side length was approximately 600 nm, and the diameter was about 50 nm.
The Raman spectra of the produced BaWO4 nanorods with a ration value of 4:6 was shown in Figure 4(a). The peaks at 926.5, 830.7, 794.6 and 330.6 cm−1 belongs to vibration mode of ν1 (Ag), ν3 (Bg), ν3 (Eg) and ν2 (Ag), respectively. Furthermore, a strong vibration at 925 cm−1 was observed, indicating the BaWO4 nanorods is good at stimulating Raman scattering in transient and steady-state [2] . This made it as a promising material for laser with self-raman conversion of radiation inside the active medium. The blue emission from BaWO4 materials has been reported at low temperatures in the literature [18] [19] [20] . Interest- ingly, as shown in Figure 4(b), a broad emission band extended from 396 nm to 498 nm (peaked at approximately 425 nm) was observed when the BaWO4 nanorods excited at 270 nm. The blue emission from BaWO4 nanorods is known to be due to radiative transitions within 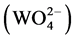 molecular complexes. Hence, a similar mechanism for tungstate materials has been reported in the literature [8] [21] [22] [23] .
molecular complexes. Hence, a similar mechanism for tungstate materials has been reported in the literature [8] [21] [22] [23] .
4. Conclusion
In the present study, BaWO4 nanoparticles have been prepared in water-in-oil (W/O) microemulsion systems composed of 1,4-dioxane and water with varied volume ratios. The results of typical TEM images indicated that the concentra-

Figure 3. The TEM (a) and SEM (b) images of the produced BaWO4 with a ratio of 1,4- dioxane and water for 4:6.

Figure 4. The Raman spectra of produced BaWO4 nanorods with a ration value of 1,4-dioxane and water for 4:6 (a), and its emission spectra at an excitation wavelength of 270 nm (b).
tion of 4-dioxane played a crucial role in the formation of BaWO4 nanorods with different morphologies. Thus, the addition of 1,4-dioxane should be controlled. Furthermore, the Raman spectra of the produced BaWO4 nanorods indicated that it is a promising material for laser with self-raman conversion of radiation inside the active medium.
Acknowledgements
This work was financially supported by the Natural Science Foundation of Jiangsu Province (13KJB150023, 15KJB210003, BK20150976), and the Natural Science Foundation of China (41501239).
Highlights
BaWO4 nanorods have been successfully synthesized.
The ratio of 4-dioxane and water played the key role.
The bands of produced BaWO4 nanorods vibrated strongly at 925 cm−1.
Cite this paper
Zhang, J., Zhu, X.S., Huang, H.Y. and Zhang, Y.P. (2017) Synthesis of Crystallized BaWO4 Nanorods in a Microemulsion System. Advances in Chemical Engineering and Science, 7, 228- 234. https://doi.org/10.4236/aces.2017.72017
References
- 1. Zhou, G., Lü, M., Gu, F., Wang, S. and Xiu, Z. (2005) Morphology-Controlled Synthesis of BaWO4 Nanocrystals via a Surfactant-Assisted Method. Materials Letters, 59, 2706-2709.
https://doi.org/10.1016/j.matlet.2005.03.054 - 2. Vodchits, A.I., Orlovich, V.A., Apanasevich, P.A., Basiev, T.T. and Zverev, P.G. (2007) Nonlinear Optical Properties of BaWO4 Crystal. Optical Materials, 29, 1616-1619.
https://doi.org/10.1016/j.optmat.2006.08.005 - 3. Byrappa, K. and Yoshimura, M. (2013) Handbook of Hydrothermal Technology. 2nd Edition, William Andrew Publishing, Oxford, 615-762.
https://doi.org/10.1016/B978-0-12-375090-7.00010-4 - 4. Hong, K., Xie, M., Hu, R. and Wu, H. (2006) Synthesis of Potassium Tungstate Micro-Walls by Thermal Evaporation. Journal of Crystal Growth, 295, 75-78.
https://doi.org/10.1016/j.jcrysgro.2006.07.002 - 5. Jia, G., Huang, C., Li, L., Wang, C., Song, X., Song, L., Li, Z. and Ding, S. (2012) Hydrothermal Synthesis and Luminescence Properties of Uniform BaMoO4:Ln3+ (Ln = Eu, Tb, Dy, and Sm) Microspheres. Optical Materials, 35, 285-291.
https://doi.org/10.1016/j.optmat.2012.08.021 - 6. Cui, C., Bi, J., Gao, D. and Zhao, K. (2008) Morphology and Crystal Phase Control in Preparation of Highly Crystallized BaWO4 Film via Galvanic Cell Method. Journal of Alloys and Compounds, 462, 16-19.
https://doi.org/10.1016/j.jallcom.2007.08.013 - 7. Byrappa, K. and Yoshimura, M. (2001) Handbook of Hydrothermal Technology. William Andrew Publishing, Norwich, 618-690.
https://doi.org/10.1016/B978-081551445-9.50009-X - 8. Pontes, F.M., Maurera, M.A., Souza, A.G., Longo, E., Leite, E.R., Magnani, R., Machado, M.A.C., Pizani, P.S. and Varela, J.A. (2003) Preparation, Structural and Optical Characterization of BaWO4 and PbWO4 Thin Films Prepared by a Chemical Route. Journal of the European Ceramic Society, 23, 3001-3007.
https://doi.org/10.1016/S0955-2219(03)00099-2 - 9. Wang, X., Xu, H., Wang, H. and Yan, H. (2005) Morphology-Controlled BaWO4 Powders via a Template-Free Precipitation Technique. Journal of Crystal Growth, 284, 254-261.
https://doi.org/10.1016/j.jcrysgro.2005.06.045 - 10. Luo, Z., Li, H., Xia, J., Zhu, W., Guo, J. and Zhang, B. (2007) Controlled Synthesis of Different Morphologies of BaWO4 Crystals via a Surfactant-Assisted Method. Journal of Crystal Growth, 300, 523-529.
https://doi.org/10.1016/j.jcrysgro.2006.12.031 - 11. Luo, Y., Tu, Y., Yu, B., Liu, J., Li, J. and Jia, Z. (2007) Synthesis of Hierarchical Barium Tungstate Corns and Their Shape Evolution Process. Materials Letters, 61, 5250-5254.
https://doi.org/10.1016/j.matlet.2007.04.010 - 12. Zhang, X., Xie, Y., Xu, F. and Tian, X. (2004) Growth of BaWO4 Fishbone-Like Nanostructures in w/o Microemulsion. Journal of Colloid and Interface Science, 274, 118-121.
https://doi.org/10.1016/j.jcis.2004.01.048 - 13. Liu, J., Wu, Q. and Ding, Y. (2005) Morphologies-Controlled Synthesis of CaWO4 Crystals by a Novel Supramolecular Template Method. Journal of Crystal Growth, 279, 410-414.
https://doi.org/10.1016/j.jcrysgro.2005.01.109 - 14. Li, D., Wu, H., Li, Z., Cong, X., Sun, J., Ren, Z., Liu, L., Li, Y., Fan, D. and Hao, J. (2006) Multi-Phase Equilibrium Microemulsions-Based Routes to Synthesize Nanoscale BaWO4 Spheres, Cylinders and Rods. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 274, 18-23.
https://doi.org/10.1016/j.colsurfa.2005.08.033 - 15. He, J., Han, M., Shen, X. and Xu, Z. (2008) Crystal Hierarchically Splitting in Growth of BaWO4 in Positive Cat—Anionic Microemulsion. Journal of Crystal Growth, 310, 4581-4586.
https://doi.org/10.1016/j.jcrysgro.2008.08.001 - 16. Chen, D., Shen, G., Tang, K., Zheng, H. and Qian, Y. (2003) Low-Temperature Synthesis of Metal Tungstates Nanocrystallites in Ethylene Glycol. Materials Research Bulletin, 38, 1783-1789.
https://doi.org/10.1016/j.materresbull.2003.09.004 - 17. Fan, W., Song, X., Sun, S. and Zhao, X. (2007) Microemulsion-Mediated Hydrothermal Synthesis and Characterization of Zircon-Type LaVO4 Nanowires. Journal of Solid State Chemistry, 180, 284-290.
https://doi.org/10.1016/j.jssc.2006.10.019 - 18. Ge, W., Zhang, H., Wang, J., Liu, J., Li, H., Cheng, X., Xu, H., Xu, X., Hu, X. and Jiang, M. (2005) The Thermal and Optical Properties of BaWO4 Single Crystal. Journal of Crystal Growth, 276, 208-214.
https://doi.org/10.1016/j.jcrysgro.2004.11.385 - 19. Tyagi, M., Sangeeta and Sabharwal, S.C. (2008) Luminescence Properties of BaWO4 Single Crystal. Journal of Luminescence, 128, 1528-1532.
https://doi.org/10.1016/j.jlumin.2008.02.006 - 20. Chen, L., Gao, Y. and Zhu, J. (2008) Luminescent Properties of BaWO4 Films Prepared by Cell Electrochemical Technique. Materials Letters, 62, 3434-3436.
https://doi.org/10.1016/j.matlet.2008.02.083 - 21. Rangappa, D., Fujiwara, T. and Yoshimura, M. (2006) Synthesis of Highly Crystallized BaWO4 Film by Chemical Reaction Method at Room Temperature. Solid State Sciences, 8, 1074-1078.
https://doi.org/10.1016/j.solidstatesciences.2005.12.013 - 22. Basiev, T.T., Sobol, A.A., Voronko, Y.K. and Zverev, P.G. (2000) Spontaneous Raman Spectroscopy of Tungstate and Molybdate Crystals for Raman Lasers. Optical Materials, 15, 205-216.
https://doi.org/10.1016/S0925-3467(00)00037-9 - 23. Zhang, X., Liao, J.Y., Yin, Z.W. and Wu, X.J. (2004) Improving Radiation Stability of Yttrium Ions Doped PbWO4 Crystals by Stoichiometric Tuning. Chemical Physics Letters, 383, 245-250.
https://doi.org/10.1016/j.cplett.2003.11.020


