Advances in Bioscience and Biotechnology
Vol.4 No.3(2013), Article ID:28614,14 pages DOI:10.4236/abb.2013.43042
An assessment of factors leading to the decline of Beclardia macrostachya (orchidaceae) population in Mauritius
![]()
1Department of Agriculture & Food Science, Faculty of Agriculture, University of Mauritius, Réduit, Mauritius
2Department of Biology and Plant Ecology, University of Antananarivo, Antananarivo, Madagascar
Email: *sudeshp@uom.ac.mu
Received 9 January 2013; revised 14 February 2013; accepted 26 February 2013
Keywords: Beclardia macrostachya; Conservation; Fertility Rate; Population Dynamics; Pollinator Limitation; Psidium Cattleianum
ABSTRACT
Clearing of forest land for agriculture and urbanization following colonization have reduced the forest cover in Mauritius to 3% of total land cover. Today exotic species such as Psidium cattleianum (wild guava), Araucaria columnaris, and Ravenala madagascarensis dominate at Pigeon Wood, the only site in Mauritius where Beclardia can be found, leaving little space for very few indigenous tree species like Labourdonnaisia glauca, Apholoia theiformis and Foetida mauritiana. Beclardia macrostachya is an orchid endemic to Mauritius, Madagascar and Reunion. Though it is abundant in the latter countries, it is one of the rarest orchids in Mauritius. An assessment of the factors associated with the stability of this orchid was carried out in forests of these three countries to understand the drastic decline of this orchid in Mauritius. Morphometric and fertility counts carried out at different forests revealed differences in fitness and fertility rates among forests of the same countries and between different countries. Stability of the different Beclardia populations was carried out based on counts of juveniles and adults. Higher fertility rates and most stable populations were observed in the forests of Reunion Island (Bebours) and Madagascar (Ambohitanteley), whereas very low fruit set were observed in Mauritius, unless manual pollination was carried out. Microscopic analysis revealed the presence of pelotons of endomycorrhiza during the early development of the seedlings, in vivo. Based on comparative studies among the different sites, pollinator limitation and absence of suitable hosts were the primary factors that led to the present decline in Mauritius. The high density of wild guava, Psidium cattleianum, which has slowly taken up most of the forest areas in Mauritius has affected the regenerating capacity of Beclardia macrostachya and the density of putative hosts of this orchid. GC-MS/MS analysis of nectar revealed α-Dglucopyranose to be the primary reward of the pollinators and benzyl alcohol, methoxybenzyl alcohol and methoxy methyl phenol to be components floral fragrance.
1. INTRODUCTION
Beclardia macrostachya, is one of the rarest orchid in Mauritius. Such a low population of this orchid requires urgent measures to develop conservation strategies for propagation. However, conservation of any taxon requires information about the ecogeographic structure of the target taxon and such data should include accepted taxon name (taxonomic and vernacular), target area where reported, flowering and fruiting timing, habitat preference, topographical preference, soil performance, geological preferences, climate and micro-climate preferences, breeding system, genotypic and phenotypic variation, biotic interactions, archelogical evidence, ethnobotanical evidence and conservation status [1]. Before starting any conservation strategy certain biological factors that govern the growth of the target taxon should also be evaluated such as spatial distribution of diversity, ecological relationships, altitude, rainfall levels, and even human factors such as movements that leads to disturbed habitat. Assessments should be carried out in the different populations present at different localities to study the changes that are occurring and also the significance of these changes in terms of viability, performance and survival of the species. Thus optimal conditions for stable populations can be evaluated, and then artificial simulations created for conservation of the species. This study aimed at defining the different factors affecting the stability of the Beclardia macrostachya populations and consequently simulating optimal conditions for the conservation of this orchid species in Mauritius.
The flora and fauna in the islands form unique communities. Island communities need not face severe competitors but sudden climatic changes, such as cyclones, would greatly affect the populations within a short time span. Developing a model for an island community would involve factors like immigration and extinction of different species with time. However, factors that would affect the number of species at a particular time will include the island size and its remoteness. The survival of a species depends on its interactions with the environment, its niche. A study of the existing populations helps to understand the factors that influence the increase in population size which itself will depend on several factors. The niche has been defined as a volume in hyperspace of which the axes are the relevant variables in the life of a species [2]. Each point represents a combination of environmental factors that permit the species to survive and a set of all such points is the fundamental niche. Therefore, a set of factors at an adequate level that permit the survival and continuity of a species was considered to be the niche breadth. Factors such as high fertility rate and favorable climatic conditions tend to broaden the niche breadth and while factors like pollinator limitation, invasion by a predator/disease narrow the niche breadth of the same species. The overall niche breadth would be defined as measures of population size, performance of populations, the fertility rate and survival rate of seedlings. Thus, the niche breadths or the set of factors regulating the stability of Beclardia macrostachya was assessed with respect to its geography, presence of a suitable pollinator, floral characters, fruit set, presence of suitable hosts, mycorhizal associations and negative effects of invasive plants.
Although most orchids provide floral reward to their pollen vectors, an estimated one third of all orchid species are pollinated without offering any reward to their visiting insects [3]. Nectar properties tend to be similar for plants visited by the same kinds of pollinators, and phenolic substances are also relatively common scent products of flowers that serve to attract pollinators or repel nectar thieves [4]. These phenolic compounds and their derivatives play a major role in the first stage of pollination, because of their well established role to have an ability to allure insects [5]. Gas chromatography-Mass spectrometry (GC-MS) analysis have been used to detect nectar and fragrant composition in orchids [6,7]. Even though flower morphology, color, and odor are selective criteria for pollinators, pollinator behavior and morphology also impose selection on flowers for effective pollination [8]. The most obvious type of nectary is the nectar spur, which arises from one of the perianth segments, but in many moth pollinated orchids (Aerangis Rchb. f., Angraecum Bory, Gymnadenia R. Br. and Mystacidium Lindl.), the spur nectar arises as an outgrowth from the proximal part of the labellum [9]. Beclardia macrostachya exhibit typical sphingophilous syndrome with white, spurred flowers and producing a strong scent [10,11]. Nevertheless, it has also been reported that grey white eye (Zosterops olivaceus) and olive white eye (Z. borbonicus) are the most probable pollinators of Beclardia macrostachya in Reunion Island [12].
Orchid seeds are minute (0.1 mm - 0.25 mm long), exalbuminous consisting of little food reserves for the developing embryo. Germination of orchid seeds require ideal conditions of light, moisture and warmth and must also be infected immediately with a mycorrhizal fungus from the soil/host plant for its survival. Mycorrhizae is a mutualistic association between soil-borne fungi and the root of higher plants and these fungi play a key role in the ecological functioning of many terrestrial ecosystems. Following seedling infection by ectomycorrhizal it was demonstrated that performance (weight & leaf nitrogen content) of the plant was dependent upon specific mycorhizal species that infected them [13]. Earliest classification based on the root fungus association classified mycorrhizae as endomycorhizae and ectomycorhizae. Presently mycorrhizae have been classified into seven groups [14] which are: 1) vesicular arbuscular (VAM); 2) ericoidmycorrhizae; 3) monotropoid mycorrhizae; 4) arbuloid mycorrhizae; 5) ectomycorrhizae; 6) ectendomycorrhizae and 7) orchid mycorrhizae. Mycorhizal associations are found throughout the orchid family. In orchid 2 types of mycorrhizal colonization may occur: 1) seed & seedling germination and 2) new roots.
All orchids utilize fungi to initiate seed germination and seedling development and availability & infection by mycorrhizae is an absolute part of an orchid life cycle, in vivo. Following dispersal of the orchid seed, if the latter lands on a suitable substrate, germination starts when the fungus penetrates the seed testa and invades the embryo. Fungal cells infect cortical cells, forming mass of tightly interwoven coils called pelotons. Pelotons are digested by host cells releasing carbohydrate from the hyphal cells [15]. Pelotons are considered to be the most distinguishing characteristic of orchid mycorrhizae and they mostly belong to the genus Rhizoctonia [15-18].
This study focused on five main criteria for the assessing the biology of this orchid: 1) Population dynamics—Population census gives relevant data about the existing populations, and successive census data can be used to assess the population tendencies. Whether the population is stable, shifting towards a decline or towards an increase can thus be measured with time. Abundance is measured as frequency of individuals over a defined unit area, at a certain time. Data of local abundance over time can be used to predict likelihood of extinction which either occurs due to deterministic (predictable) and stochastic (random) processes or a combination of both [19]. Factors affecting the population size can therefore be evaluated through analysis of different populations at different geographical locations or with different biotic and abiotic factors under the study area; 2) Spatial distribution—This aspect defines the different patterns in which the plants are distributed in the area. It gives an idea whether the plants are clustered together into a single community, or distributed as patches over the area, or scattered evenly in the area. The spatial distribution will affect processes like pollination, dispersal, colonization. A singly clustered population is prone to extinction if the area is affected by certain calamities such as a cyclone, compared to a scattered population. However, within a clustered population pollination success will be much higher compared to scattered individuals. Within an area the plants may have the same density but need not have the same distribution pattern; 3) Performance of the species—It is necessary to know how well a plant is growing in a particular area and a measure of some suitable character can often be made, which reflects the relative vigour or performance of an individual. Suitable measures to assess the performance, include leaf length, leaf width which gives an idea of the leaf area available for photosynthesis; flower number, number of seeds per capsule, fertility rate measures that represent the reproductive capacity of the individuals. Choice of character depends on species under investigation but, generally, leaf length and fertility rate is suitable and gives a good measure of performance. Thus, general vigour under relatively different environmental conditions could be determined in the fields; 4) Phenology and breeding System—Phenology describes the flowering seasons of an orchid while the breeding system describes the fruiting ability of a population with respect to parental types involved in successful pollination. Phenology includes factors that govern the flowering ability like vernalisation or day-length variation at different time of the year. Phenological variations are more dependent on the climatic conditions. In contrast, the breeding system is dependent on factors that are present in the plant itself, such as ability to self-fertilise or cross-fertilise. Studies of the breeding system include determination of the mating types; 5) Mycorhizal associations—it is important for the successful germination and growth of orchid seedlings.
2. MATERIALS AND METHODS
2.1. Data Collection and Field Surveys and Assessment of the Population Dynamics
In Mauritius, initial data were collected from the Mauritius Sugarcane Industry Research Institute (MSIRI) National Herbarium. Oldest specimens collected dated back to 1970s and recorded from different regions, such as Grand Bassin, Bois Sec and Pigeon Wood. Data were also collected from the National Parks and Conservation Services (NPCS), and with help of Forest officers of NPCS initial surveys were carried out in the different forest areas managed by NPCS. The Forestry Department (Ministry of Agro-Industry & Food Security) was also involved in the surveys in different regions of Mauritius.
Understanding the biology involved the study of different aspects of the orchid. Specific considerations were given to the associated fauna and flora (hosts and substratum) of Beclardia macrostachya. Broader studies of its ecology with more emphasis on the spatial distribution and abundance of Beclardia colonies were carried out. The initial observations were carried out along transects, but later the whole Pigeon Wood area was surveyed and each Beclardia individual was tagged and its relative position was noted on a map of the area. The relative abundance in the different forests of Reunion Island and Madagascar were also studied. Quadrats (1 m2) were set randomly to estimate the relative abundance of the wild guava in the Mauritian forests, and transects were used to estimate the abundance of Beclardia macrostachya in the different forest.
Four sites were selected from Reunion Island which were Piton Bebours (1800 m), Foret d’Eden (1300 m), Tacamaca (900 m) and Radiers (600 m). All these forests were at different altitudes. From Madagascar, the National Park of Andasibe Mantadia (Crete d’Ambatomandondona, alt: 922 m) and Ambohitantely Nature Reserve (2 sites: slope 1300 m and forest boundary: 1500 m) were studied for the survey (Figures 1(a)-(d)).
2.2. Assessment of Performance
In the experiments carried out the length of the longest leaf was measured to the nearest mm. The second criteria for assessing the performance, was through the reproductive success of the species. For this experiment the fertility rate or fruiting index was measured, as ratios of the flower to fruit conversion. The number of capsules per flower stalk was determined, and the scars left (bract) after the flowers had shed off were considered as flowers which failed to produce capsule. Thus the total number of flowers and fruits per inflorescence were counted. Records of every individual that flowered were taken. Thus, the fruiting index was calculated as the fraction of fruit developed over the total number of flowers per inflorescence. The number of flower per inflorescence gave an idea of the robustness and the genetic inheritance of the populations, while fertility rate was more environmentally dependant (pollinator efficiency).
2.3. Demographic Studies
The stability of different Beclardia macrostachya popu-
 (a)
(a) (b)
(b) (c)
(c)
Figure 1. Distribution of Beclardia macrostachya: (a) Madagascar two sites; (b) Reunion Island five sites; (c) Mauritius one site; (d) Distribution of Beclardia macrostachya patches at Pigeon wood (Mauritius).
lations were studied at different locations based on the counts of plantlets, juveniles and adults, based on the fact that stable populations tend to have more juveniles than adults. The Beclardia patches were assessed within 10 m2 quadrats within which counts were made for the number of putative host plants (indigenous tree species, number of wild guava plants and other exotic plant species. For the wild guava density, 2 sites were chosen randomly and several 1 m2 quadrats were set to count the number of wild guavas and indigenous tree species.
2.4. Microscopic Observations
Plantlets of Beclardia macrostachya were collected from the wild and brought to the lab. The plantlets were placed in paper envelopes and oven dried for 5 hours at 65˚C. The roots were then carefully chopped using a sharp blade and mounted on slides. Permanent slides were also prepared using Canada balsam. The slides were observed and digital photographs captured under a high power inverted microscope (Leica DMIL).
2.5. Qualitative Analysis of Sugars & Aromatic Compounds in Flower
It is often observed that insect pollinated flowers (moth pollinated) are highly scented and chemically consist of aromatic compounds. Thus, the nectar and floral parts were analysed to determine the presence of aromatic compounds. Initially a very thin needle and syringe was used to collect the nectar but the volume collected per flower was less than 2 μl. Thus, the flower were sliced/ cut open through the nectaries using a sterile sharp blade and were soaked in a solvent (MS grade methanol), followed by sonification for 1 hour for effective extraction. The extract was allowed to concentrate and then injected in GC-MS/MS (Gas Chromatography-Mass spectrometry Mass Spectrometry). GC-MS/MS was run 3 times with sub-samples of the extract to verify consistency of chemicals detected. The data were then input in the GC-MS/ MS library to determine the presence of aromatic compounds and sugars. Once the chemicals were known, their chemical structures were generated from ChemOffice 2004.
2.6. Floral Morphometry and Breeding System
Flowers were placed on graduated paper and digital photographs of flowers were taken using a Canon Powershot A470. Using the software scion Image Alpha 4.0.3.2, the length of eperon & spur, labellum and nectar diameter were calculated as shown in Figure 2. With the assumption that the length of the beak and the length of the eperon (floral tube to nectar) should be complementary so as to ensure pollination, measurements of beak length and tongue length of 3 species of Zosterops encountered in Mauritius were recorded. Available data and measurements for these were kindly provided by Dr. Nicholas Zuel of the Mauritius Wildlife Foundation. Flowers were manually self pollinated and cross-pollinated but limiting the number of fertilized flowers per inflorescence to 3, with the fact that in most orchid species, the probability of a flower to produce a fruit decreases if many flowers are pollinated because enough resources is not available for fruit maturation [20].
2.7. Statistical Analysis
Statistical analysis was carried out using the software SPSS 13.0. The readings were given as means ± SE for leaf diameter, floral counts and fruiting index. In order to ensure minimal error, temporal data were collected during 2 years at Bebours, Takamaka and Eden. Anova was carried out to determine significant differences between fruiting index and floral counts at the same locality but at different flowering periods (2005, 2006 and 2010). Multiple comparative studies were carried out following Anova using Tukey HSD and the harmonic mean Sample size to determine differences in performance of Beclardia macrostachya at different locations. For the wild guava populations, relative abundance was calculated as number of individuals per meter square.
3. RESULTS
3.1. Field Survey and Data Collection from the National Herbarium in Mauritius
The preserved specimen of Beclardia macrostachya were analysed. It was reported that the plant is herbaceous with an epiphytic habit, and were commonly occurring on the trunk of Labourdonnasia sp. They were also reported to be quite common in the areas near Grand Bassin, Bois Sec and Chemin Cheval. They were also reported from Plaines Paul and Pigeon Wood. Since all the specimens bearing flowers were collected in the months of March and April, it could be said that these were the months when this orchid flower. Field surveys that were carried out at different forest areas covering the whole of Mauritius. However, this orchid was found only at one region, the forest of Pigeon Wood. The orchids commonly sharing the habitat of Beclardia included Oberonia sp., Jumellea sp., Polystachya sp., and Angraecum pectinatum.
Nine such areas have been found within Pigeon Wood, where the orchids occur in small populations. Extensive surveys of the forest areas were carried out based on phytogeographical studies. Areas that show similar climatic condition as Pigeon Wood were included. By March 2010, 82 individuals were recorded from Pigeon Wood and most of them tagged and the populations were monitored by weekly visits.

Figure 2. Measurement of floral morphology.
3.2. Study Area in Mauritius (Pigeon Wood)
Pigeon Wood is a small forest area managed by the National Parks and Conservation Services Mauritius and, so far, this has been the only place from where Beclardia has been presently reported. The area distributes itself along a sloping area, 550 m altitude. It is part of the super humid zone, with often rainy and misty mornings. The forest area is dominated by introduced tree species like Araucaria, Eucalyptus, Callistemom citrinus, Ravenalla madagascarensis and the wild guava (Psidium cattleianum). However, some native plants like the Labourdonaisia glauca, Apholoia theiformis, Foetida mauritiana are also present. The tree fern Cyathea excelsa is also very common in this area. This area is also a breeding place for the very rare endemic bird, pink pigeon. Zoosterops barbonicus are also quite common in this place. Monkeys, rats and wild boars are very common in this area.
3.3. Associated Fauna and Flora
Being an epiphyte, suitable hosts need to occupy the forests area for the survival of this orchid. Beclardia was found to grow from 4 m up to 12 m above the ground level. The putative hosts bear moss and lichens growing along the branches and the bark were persistent. Pigeon Wood was predominantly dominated by Araucaria, Eucalyptus and wild guava, and these tree species however do not represent good hosts. Beclardia is found mostly on the native species such as Labourdonnaisia glauca, Foetida mauritiana and Mimusops sp. Beclardia roots are often seen anchored around the branches and usually covered by a layer of moss and lichens. Occurrence of mosses and lichens as substratum is common feature.
The associated fauna comprise of pollinators and predators of this orchid. On the Beclardia leaves, insect galls were observed. Snails were also seen to be feeding on the young leaves. At the end of the fruiting season of wild guava, few tagged Beclardia individuals were seen to have been totally eaten up by monkeys. All the leaves were eaten up and only the roots and small portions of the stem were left. The birds that were observed in this forest area include the Mauritius grey white eye (Zoosterops barbonicus), Mauritius olive white eye (Zoosterops Chloronothus), Paradise Flycatcher (Terpsiphore boubonnensis desolata) and the famous Pink pigeon (Nasoenas mayeri). Flies are also found in this region.
3.4. Mycorhizza
Microscopic studies revealed presence of Mycorhiza (pelotons) in the roots of plantlets of Beclardia macrostachya seedlings (Figure 3). This suggest that presence of mycorhiza may have an important role in the germination of the exalbuminous seeds of this orchid Surveys showed the occurrence of this orchid only at Pigeon Wood. Beclardia macrostachya were not found from places where it was earlier reported. Almost all the forest areas in Mauritius were surveyed but, even though other orchids were reported Beclardia macrostachya was not found. Other orchids sharing common habitat with Beclardia include Oberonia sp., Angraecum pectinatum, Polystachya sp. and Jumellea sp. At the beginning the forest officers reported only 2 known individuals and a probable estimate of a population of 15 individuals was given by Roberts [21]. As the survey progressed more individuals were encountered and the actual population recorded was around 75 individuals (January 2006) and 82 individuals (2010). Beclardia seemed to occur in very

Figure 3. Tranverse section of Beclardia macrostachya (seedling) root to show presence of mycorrhiza pelotons Spatial distribution.
small populations at specific areas. They were distributed into smaller populations within a limited area, rather than between distributed evenly in the forest. Eight such patches have been observed and some individuals have been found randomly, especially along the river (Table 1).
3.5. Phenology and Breeding
Beclardia macrostachya flowers from January to June. The breeding experiment was successful and fruit set was obtained from both self and cross pollinated flowers.
3.6. Performance of the Species
Observations of the different individuals suggest that each colony did not share the same developmental stage of the different individuals. A few individuals were older, evident by higher number of leaves, bigger in size and ability to produce inflorescence. The plants that flowered rarely bear more than 8 leaves, and a few individuals did produce more than one inflorescence at a time, but never more than 3 within the same flowering season. The performance of the Mauritian species was thus measured with respect to comparative leaf length.
The developmental stages of the individuals were determined based on leaf length measures, ability to flower and also the number of leaves present. Individuals with 6 or more leaves were considered adults. The persistent flower stalks of Beclardia helped both in the identification of Beclardia individuals and also to account for the developmental stage. Only 19 individuals were found to be juvenile and 46 could be considered as adults, which indicated a higher proportion of adults than juvenile plants in the population. Leaf length measures were not suitable for assessing the productivity, because of the varying developmental stages of the plant, and these measures were used to assess the developmental status of the individuals along with results from the leaf number and flowering reported per individual.
3.7. Psidium cattleianum Density around Beclardia Patches and Other Sites
The density of wild guava was very high compared to putative hosts (Table 2) at different Beclardia patches. This density of wild guava was observed throughout the forest with an average of more than 20 wild guava individuals/m2. Thus, probability of the orchid seeds to fall on wild guava was much higher, and consequently lower germination.
Table 1. Site assessment of Beclardia macrostachya populations and associated flora at Pigeon Wood.

*Indicates site where successful breeding experiment was carried out and the resulting capsules were used for in vitro culture.
Table 2. Wild guava population at Pigeon wood.

3.8. Studies at Reunion Island
The four different forest areas were selected based on the fact that they were at different altitudes and Beclardia macrostachya were reported in these areas. The Radiers (400 - 600 m) an area comparable to Pigeon Wood Mauritius, low altitude forests. Tacamaca (600 - 800 m) represented a medium altitude forest, while Eden (1600) and Bebours (1800 m) were at higher altitudes. Each area represented different climatic conditions, resulting in differences in the vegetations.
At Reunion Island, temporal data indicated a certain stability of the Beclardia populations, and the fertility rates indicate that the pollinator is very efficient and there is no major concern regarding fertility rates (Figure 4). However, the wild guava has slowly crawled inside the forests areas of Eden, Tacamaca and also part of Piton Bebours. It is tough for the wild guava to compete with Acacia heterophyllum which takes major regions at Bebours and ensures a suitable host for Beclardia macrosstachya. However, at Eden and Tacamaca the wild guava is gaining much ground, and may eventually contribute to the decline in Beclardia macrostachya and other epiphytic orchids populations.
The temporal data from Reunion Island (Bebours) serve as a control/model for the stability of Beclardia macrostachya. Geographical and biological dynamics that deviate from this ecosystem, will reflect a deviation from optimal conditions of this orchid. Therefore, the Mauritian population represents a disturbed niche for Beclardia macrostachya and tending towards extinction, unless restoration measures are taken.
3.9. Studies at Madagascar
Population count of Beclardia macrostachya at Andasibe was low and the individuals were scattered, whereas at Ambohitantely (site1, 1500 m Alt.) a very dense population was observed near the forest boundary, whereas along the slope at Ambohitantely the individuals were very scattered and occupied the taller regions of the forest strata. There was a wide diversity of host plants for

Figure 4. Fertility rate at three different forest sites of Reunion Island.
Beclardia at Andasibe whereas, at Ambohotantely (1500 m) Owapaka densiflora was the dominating host plants.
The average number of flowers was 6.8 ± 2.4 at Andasibe and 7.7 ± 3.16 at Ambohitantely, there seem to be 2 types of individuals. The first group of individuals bearing shorter leaf length (<12 cm) and a floral count ≤ 5 per inflorescence, and the second group that generally bear longer leaves (>12 cm) and floral count ≥6. These measures indicated that the plants at Andasibe were a different ecotype, which was earlier described by Bosser [22] as a different species (Beclardia grandiflora).
Fertility rate was low at both sites in Madagascar, 27.1% at Andasibe and 32.3% at Ambohitantely. These fruiting data could not be used for statistical analysis because very few individuals with capsules were observed. Madagascar describes 2 niche systems for Beclardia macrostachya, one that is more stressed at Andasibe, and the other more stable population at Ambohitantely.
Comparative fertility rates among the three countries indicate high fruiting success in Forest of Reunion Island and very low fruit set in Mauritius and at Andasibe (Madagascar). Adults leaf length measurements indicates that the Mauritian populations have the shortest leaves (p < 0.05), and Andasibe (Madagascar) populations with longest leaves (p < 0.05). The Floral count showed no significant differences among the different populations (Table 3).
3.10. Nectar Components-Sugars and Aromatic Compounds
Three sugars were detected: DL Arabinose, D(+) Talose and alpha-D-Glucopyranose (D-glucose). Eight aromatic compounds were detected: 4-(methoxymethyl)-phenol, 2-Methoxybenzyl alcohol, 2-Methoxy-4-Vinylphenol, 4- hydroxy-benzenemethanol, salicin, Desulphosinigrin, Felbamate and 1,8-Diazacyclotetradecane-2,7-dione (Figure 5).
4. DISCUSSION
4.1. Biological Implications
It is often assumed that healthy populations follow a J shaped distribution where adults are outnumbered by saplings, while populations with few juveniles relative to adults are in decline [23]. In Mauritius, 29 % of the individuals are juvenile and in the past years very few fruiting success recorded. As per Levin’s model [24], extinction being a function of population size and the fact that large patches with large populations being less likely to become extinct, this small population of this orchid in Mauritius is much likely to become extinct unless recolonisation (rescue effects) is carried out through conservation strategies.
Table 3. Assessment of performance at Reunion Island, Madagascar and Mauritius based on field counts. 
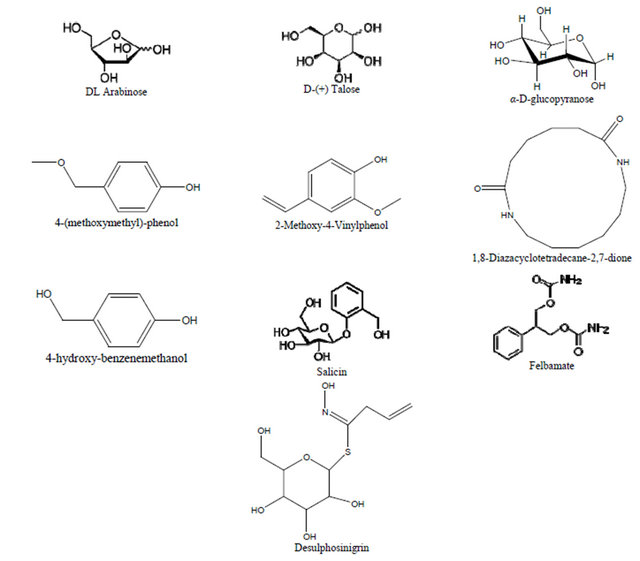
Figure 5. Components detected by GC MS/MS analysis and their structural formula.
Beclardia macrostachya was found to be self and cross pollinating, but requires a suitable vector for pollination. The low fruiting success in Mauritius can be attributed to the limitation of a suitable pollinator. Several orchids have been found to be self-compatible, but still completely dependent on vectors for pollination for ex: Brownlea coerulea, B. macroceras and B. parviflora, [25], Epidendrum secundum [26], Epipactis consimilis [27], Habenaria paviflora [28] and Holcoglossum rupestre [29].
All the flowers that were manually pollinated developed fruits and this confirmed the fact that pollinator limitation, has been the major factor in the decline in the Mauritian population. The scattering of the individuals in scarce patches have also made them less conspicuous to pollinators, and thus reduced pollination efficiency [30]. Of the few capsules that developed, it was found these individuals were present in host plants that were exposed on tall host plants along the river cliffs/sides (Table 1: sites 6, 7, 8 and 9). These exposed individuals were more visible to pollinators.
The effects of the varying altitude could be clearly observed through the abundance and fertility rates, with the orchid showing higher fertility rates and abundance at high altitudes region like Bebours and Eden at Reunion Island, Ambohitanteley at Madagascar. Higher fertility rates would be credited to the presence of very efficient pollinators, which might have preferentially migrated to the higher altitude forests where there is lesser disturbance, but also a much healthier habitat.
The size of the plants were different among the populations in Reunion Island itself, the more robust individuals recorded at Eden and Bebours, and least Robust individuals found at Radiers and Mauritius. This can be reflected as the performance of the species at varying altitudes or climatic conditions. A general trend was observed with respect to leaf length and flower number, the longer the leaves, the greater was the number of flower bearings by the inflorescence. However, at Tacamaca and Bebours the same trend was not observed, with Tacamaca showing no corelation between the two and this was explained by the fact that the leaves were long (9 cm), which meant that all the plants that were flowering at Tacamaca had the longest leaf above 9 cm, while at other regions like Bebours and Eden an individual with the longest leaf around 3 - 5 cm was able to flower. This explains the fact that at higher altitudes this orchid performed better both in terms of productivity and fertility rates [31].
Madagascar with a rich orchid flora of about 1000 species and with 90% of its orchid species being endemic, many of these species are threatened by the rapid rate of environmental change in the island through deforestation making place for agriculture [32]. Our studies from Madagascar also show a preferential adaptation of Beclardia macrostachya to higher altitude regions. Wild guava has also been noticed along the track to Lekato (Madagascar), which implies that authorities should get vigilant about the widespread of this invasive species.
The negative impact of wild Guava in Mauritius was very much obvious in Mauritius with a negative co-relation between wild guava and putative hosts. In Reunion Island at Eden, parts of this forest were invaded by wild guavas, and seedlings of Beclardia macrostachya were seen on the ground, due to the shedding of the bark of wild guava. Wild guava directly prevent the growth of putative hosts of Beclardia and the guavas themselves help in reducing the population of Beclardia by capturing the dispersed seeds, but not allowing them to develop further beyond the seedling stage through shedding-off of their outer bark.
Results from GC-MS MS analysis indicate that this orchid offers nectar as a reward. Alpha D glucopyranose which is the major soluble form of sugar in plants is sought to be the major component of the reward in the nectar. Sucrose was not detected at all in the nectar, this has also documented by Wenzler [33]. Talose (a rare sugar) and arabinose were reported to be a component of the nectar of Brassica napa [34]. Benzyl alcohol/(Benzene methanol,4-hydroxy) is known to be insect attractant and reported to be major components of floral [35-40]. Of the other aromatic compounds detected in Beclardia macrostachya, methoxybenzyl alcohol and methoxy methyl phenol are also reported to be a component of floral scents in orchids [37]. These results also describe the observation of 3 different kind of insects foraging on the flowers (Figures 6(a)-(c)) of Beclardia macrostachya and the fact that this orchid exhibit sphingophilous syndrome. Though, these insects visit the flowers of Beclardia macrostachya they may not necessarily pollinate the flowers as earlier reported by Micheneau [41] in a similar study carried out in Mauritius for an endemic orchid Angraecum cadetti.
Orchids pollinated by birds generally exhibit short spur (<2.5 cm), bear several flowers per inflorescence and are not scented. Pollination of some orchids is thought to have evolved from sphingophily (moth pollinated) to ornithophily and some have become completely independent of their insect pollinators in the mascarene islands [10]. As described from the high fertility rates observed in high altitude forest of Bebours and Eden of Reunion Island (Table 3), and the fact that most of the orchids that are pollinated by birds are found at high altitudes, it can be hypothesized that Beclardia macrosatachya must be bird pollinated in Reunion island and Mauritius. Since these Zosterops are not observed in Madagascar this orchid is insect pollinated in Madagascar, although one species Zosterops maderaspatanus feeding mainly fruit, flower buds and insects [42] is reported from Andasibe. However, this can only be confirmed by further studies in Madagascar to determine the exact pollinator.
4.2. Probable Reasons of Decline in Mauritian Population
4.2.1. Habitat Destruction and Alterations
Field surveys at Reunion Island showed flourishing and abundant populations of Beclardia macrostachya, and it was quite puzzling to sort out the reasons of the drastic
 (a)
(a) (b)
(b) (c)
(c)
Figure 6. Visitors of Beclardia macrostachya flowers. (a) A tiger moth; (b) A hemipteran bug; (c) An aphid.
decline in the Mauritian population, now to a mere 82 individuals at a single protected forest area and with an extremely low fruiting success.
The most evident fact is that, at present the Mauritian forest covers only 3% [43] of the total land area this is an indication of the ferocious deforestation which has taken place in the past years and the spread of exotic species in the remaining forest patches [44]. Beclardia was earlier reported in the areas near Grand Bassin and Bois Sec and today most of these forest areas have been cleared and there have also been an alteration of the habitat with introduction of pine trees and Araucaria. The actual habitat has now been restricted to a mere 5 km2 area at Pigeon wood. Roberts & Wilcock [45] made similar observations and also concluded that areas like Les Mares which were once populated with Beclardia macrostachya have now been cleared and reported that this orchid showed no fruiting success for the period between 1997 to 2001, suggesting a lack of recruitment from seeds.
The invasive wild guava did have an additive negative effect on the Beclardia populations. This invasive plant primarily prevents the growth of putative hosts for Beclardia. The Mauritian forests are all invaded by this plant and it grows so fast and propagates so quickly that it has become difficult to control. Only strict and meticulous clearing can stop its widespread. But, even animals (wild boars) act as vectors for the dispersal of the seeds, such that even newer areas slowly get invaded. If the wild guava could be suitable hosts for these orchids maybe it would have had some positive impact, but evident from field observations at Eden Forest (Reunion Island) seedlings of Beclardia are seen to die off near the ground. This was the result of the shedding off of the outer bark of the wild guava (typical character of Myrtaceous plants), and along with the bark the Beclardia seedlings fell to the ground and died.
The presence of a suitable pollinator determines the fertility rate of a population. The most probable pollinator was thought to be Olive white Eye (Zosterops olivacea), a nectariferous bird [46] present both at Reunion Island and Mauritius, however, the Mauritian population of this bird is low.
4.2.2. Other Factors
Mauritius is very often exposed to strong cyclones in summer season, and such condition can wipe off a population very quickly and this orchid itself being an epiphyte will often be exposed to violent winds, and the host plants themselves often end up with broken branches or totally uprooted after a cyclone. Even droughts can have severe consequence on these epiphytes, even though they show adaptations to xerophytic conditions. Mauritius is often exposed to periods of droughts which may last for more than two years and under these conditions, such limited population would be easily wiped off. Threat from poaching is very much less now, as this orchid is present in a well monitored forest area, which has properly been fenced by the National Parks and Conservation services. After the end of the fruiting season of wild guavas, animals like monkeys are suddenly in a situation of reduced available food, and in such situations they either feed on other seeds or fruits (which become less abundant), and very often they feed on fleshy leaves. Beclardia individuals with the leaves totally eaten up by animals were observed and another orchid which often eaten up by these animals is Oberonia sp. Other animals like snails and caterpillars were also observed to feed upon the tender leaves (Figure 7) of Beclardia, which adds to more pressure on the existing populations. Thus it is not a single factor, but a whole set of smaller selective pressures like climatic conditions, grazing by animals, lack of suitable hosts, impacts of wild guava on seedling development and the absence of suitable pollinating vectors are acting together such that the orchid is becoming much more vulnerable to extinction in Mauritius.

Figure 7. Grazing of Beclardia macrostachya leaves.
5. CONCLUSION
Stable populations of Beclardia macrostachya depend on the availability of suitable host plants, suitable pollinator to ensure effective fertilization and consequent seed regeneration, and climatic conditions prevailing at higher altitude regions which are heavy rainfall and colder temperature (22˚C - 28˚C). Pollinator limitation, widespread of wild guava and limited host plants have primarily led to the decline in the Beclardia macrostachya population in Mauritius.
6. ACKNOWLEDGEMENTS
We thank Dr. Thierry Pailler of University de La Reunion, Mr. Bachraz and Mr. Mario Allet of the National Parks and Conservation Services of Mauritius and Dr. Nicholas Zuel of the Mauritius Wildlife Foundation. The Forestry and Biodiversity Departments (Ministry of AgroIndustry & Food Security, Mauritius) also helped during the surveys in the different forest areas. We also thank the Ministry of Environment, Forestry and Tourism of Madagascar for supporting our research in the Forest reserves of Ambohitantely and Andasibe. Our sincere thanks also go to Dr. Goury and Mr. Babeea for helping us with the GC-MS/ MS analysis and to Dr. S. Ganeshan of the MSIRI and Prof. S. Facknath in the identification of the insects. We are also grateful to Jacques Dielen and Francois Vandreschike for taking the pictures of the visitors of Beclardia macrostachya flowers. This research work is funded by the Tertiary Education Commission (Mauritius), and supported by the University of Mauritius.
REFERENCES
- Maxted, N. and Guarino L. (1997) Ecogeographic surveys. In: Maxted, N., Ford-Lyodand, B.V. and Hawkes, J.G., Eds., Plant Genetic Conservation, the in Situ Approach, Chapman and Hall Publication, London, 69-86. doi:10.1007/978-94-009-1437-7
- Mathew, A.L. (1995) The niche concept revisited: Mechanistic models and community context. Ecology, 76, 1371-1382. doi:10.2307/1938141
- Vereecken, N.J., Stephan, R. and Pierluigi, C. (2007) A contribution to the pollination biology of Ophrys scolopax Cavanilles (Orchidaceae) in southern France. Natural Belges, 88, 17-26.
- Nicolson, W.S. and Thornburg, R. (2007) Nectar chemistry. Springer, Berlin, 215-263.
- Jakubska, A., Przado, D., Steininger, M., Aniol-Kwiatkowska, J. and Kadej, M. (2005) Why do pollinators become “Sluggish” nectar chemical constituent from Epipactis Helleborine (L.) Cranz (Orchidaceae). Applied Ecology and Environmental Research, 3, 29-38.
- Reis, G.M., Pansarim, E.R., da Silva, U.F., do Amaral, E. and Marsaioli, J.A. (2004) Pollinator attraction devices (floral fragrances) of some Brazilian orchids. Arkivoc, 4, 103-111.
- Sadler, J.J., Smith, M.J., Zettler, W.L., Alborn, T.H. and Richardson, W.L. (2011) Fragrance composition of Dendrophylax lindenii (Orchidaceae) using A novel technique applied in situ. European Journal of Environmental Sciences, 1, 137-141.
- Schiestl, P.F. (2005) On the success of a swindle: Pollination by deception in orchids. Naturwissenschaften, 92, 255-264. doi:10.1007/s00114-005-0636-y
- Davies, K.L., Stpiczy-Nnska, M. and Gregg, A. (2005) Nectar-secreting Floral Stomata in Maxillaria anceps Ames & C. Schweinf. (Orchidaceae). Annals of Botany, 96, 217-227. doi:10.1093/aob/mci182
- Micheneau, C., Fournel, J. and Thierry, P. (2006) Bird Pollination in an Angraecoid Orchid on Reunion Island (Mascarene Archipelago, Indian Ocean). Annals of Botany, 97, 965-974. doi:10.1093/aob/mcl056
- Jacquemyn, H., Micheneau, C., Roberts, L.D. and Pailler, T. (2005) Elevational gradients of species diversity, breeding system and floral traits of orchid species on Reunion Island. Journal of Biogeography, 32, 1751-1761. doi:10.1111/j.1365-2699.2005.01307.x
- Probst, J.-M. and Abhaya, K. (2003) Le nectar de Beclardia macrostachya (Orchidaceae) recherché par l’Oiseau vert Zosterops olivaceus et l’Oiseau blanc Z. borbonicus. Bulletin Phaeton, 17, 56.
- Kennedy, D.T., Hortal, S., Bergmann, E.S. and Bruns, T.D. (2007) Competitive interactions among three ectomycorrhizal fungi and their relation to host plant performance. Journal of Ecology, 95, 1338-1345. doi:10.1111/j.1365-2745.2007.01306.x
- Agarwal, P. and Sah, P. (2009) Ecological importance of ectomycorrhizae in world forest ecosystems. Nature and Science, 7, 107-116.
- Athipunyakom, P., Manoch, L. and Piluek, C. (2004) Isolation and identification of mycorrhizal fungi from eleven terrestrial orchids. Kasetsart Journal, 38, 216-228.
- Taylor, D.L. and Bruns, D.T. (1997) Independent, specialized invasions of ectomycorrhizal mutualism by two nonphotosynthetic orchids. Proceedings of the National Academy of Sciences of the United States of America, 94, 4510-4515. doi:10.1073/pnas.94.9.4510
- Kristiansen, K.A., Taylor, D.L., KjØller, R., Rasmussen, H.N. and Rosendahl, H. (2001) Identification of mycorrhizal fungi from single pelotons of Dactylorhiza majalis (Orchidaceae) using single-strand conformation polymorphism and mitochondrial ribosomal large subunit DNA sequences. Molecular Ecology, 10, 2089-2093. doi:10.1046/j.0962-1083.2001.01324.x
- Taylor, D.L. and McCormick, K.M. (2007) Internal transcribed spacer primers and sequences for improved characterization of basidiomycetous orchid mycorrhizas. New Phytologist, 177, 1020-1033.
- Gillman, M. and Hails, R. (1997) An introduction to ecological modelling: Putting theory into practice. Methods in ecology series. Blackwell Science, Oxford, 1-6.
- Mattila, E. and Kuitunen, T.M. (2000) Nutrient versus pollination limitation in Platanthera bifolia and Dactylorhiza incarnata (Orchidaceae). OIKOS, 89, 360-366.
- Roberts, D.L. (2001) Distribution of Mascarene orchid flora; Conservation prioritization. Reproductive Biology and Conservation of the orchids of Mauritius. University of Wales, Aberystwyth, 152-155.
- Bosser, J. (1997) Contribution à I’étude des orchidaceaede madagascar et des mascareignes. XXVII. Adansonia Series 3, 19, 181-197.
- Lykke, A.M. (1998) Assessment of species composition change in savanna vegetation by woody plants, size class distribution and local information. Biodiversity and Conservation, 7, 1261-1273. doi:10.1023/A:1008877819286
- Lopez, E.J. and Pfister, A.C. (2001) Local population dynamics in metapopulation models: Implications for conservation. Conservation Biology, 15, 1700-1709. doi:10.1046/j.1523-1739.2001.00140.x
- Larsen, W.M., Craig, P., Johnson, D.S. and Olesen, M.J. (2008) Comparative biology of pollination systems in the African-Malagasy genus Brownleea (Brownleeinae: Orchidaceae). Botanical Journal of the Linnean Society, 156, 65-78. doi:10.1111/j.1095-8339.2007.00725.x
- Pansarin, E.R. and Amaral, M.C.E. (2008) Reproductive biology and pollination mechanisms of Epidendrum secundum (Orchidaceae). Floral variation: A consequence of natural hybridization? Plant Biology, 10, 211-219. doi:10.1111/j.1438-8677.2007.00025.x
- Ivri, Y. and Dafni, A. (1997) The pollination ecology of Epipctis consimilis (Orchidaceae) in Israel. New Phytologist, 79, 173-177.
- Singer, B.R. (2001) Pollination biology in Habenaria parviflora (Orchidaceae) in South Eastern Brazil. Darwiniana, 39, 201-207.
- Jin, X.H., Chen, S.C. and Qin, H.N. (2005) Pollination system of Holcoglossum rupestre (Orchidaceae): A special and unstable system. Plant Systematics and Evolution, 254, 31-38. doi:10.1007/s00606-005-0310-z
- Rein, B., Hans, J. and Martin, H. (2008) Pollination efficiency and reproductive patterns in relation to local plant density, population size,and floral display in the rewarding Listera ovata (Orchidaceae). Botanical Journal of the Linnean Society, 157, 713-721. doi:10.1111/j.1095-8339.2008.00830.x
- Bhoyroo, V., Sanmukhiya-Ranghoo, V.M. and Puchooa, D. (2008) Conservation of Beclardia macrostachya. UoM Research Journal, 13A, 4-45.
- Cribb, P. and Hermans, J. (2007) The conservation of Madagascar’s orchids. A model for an integrated conservation project. Lankesteriana, 7, 255-261.
- Wenzler, M., Ischer Dirk, H., Oerther, T. and Schneider, B. (2008) Nectar formation and floral nectary anatomy of Anigozanthos flavidus: A combined magnetic resonance imaging and spectroscopy study. Journal of Experimental Botany, 59, 3425-3434. doi:10.1093/jxb/ern191
- Bender, R., Klinkenberg, P., Jiangb, Z., Bauera, B., George, K., Nguyenc, N., Pererac, M.A.D.N., Nikolau, B.J. and Clay, J.C. (2012) Functional genomics of nectar production in the Brassicaceae. Flora, 207, 491-496. doi:10.1016/j.flora.2012.06.005
- Price, D.W., Mazrimas, J.A. and Summers, F.M. (1967) Chemical attractants for navel Orangeworm moths. California Agriculture, 21, 10-11.
- Lampman, R.L., Metcalf, R.L. and Andersen, J.F. (1987) Semiochemical attractants of Diabrorica undecimpuncrara howaardi Barber, southern corn rootworm, and Diabrorica virgijera virgijera leconte, the westerncorn rootworm (Coleptera: Chrysomelidae). Journal of Chemical Ecology, 13, 959-975. doi:10.1007/BF01020175
- Knudsen, T.J., Eriksson, R., Gershenzon, J. and Stahl, B. (2006) Diversity and distribution of floral scent. The Botanical Review, 72, 1-120. doi:10.1663/0006-8101(2006)72[1:DADOFS]2.0.CO;2
- Guedot, C., Landolt, J.P. and Smithhisler, L.C. (2008) Odorants of the flowers of the butterfly bush Buddleja davidii, as possible attractants of pests species of moths. Florida Entomologist, 91, 576-582.
- Johnson, D.T., Lewis, B.A., Bryant, R.J., Liyanage, R., Lay, J.O. and Pszczolkowski, M.A. (2009) Attractants for the Green June Beetle (Coleoptera: Scarabaeidae). Journal of Economic entomology, 102, 2224-2232. doi:10.1603/029.102.0627
- Jakubsca-Busse, A. and Kadej, M. (2011) Thepollination of epipactis zinn, 1757(Orchidaceae) species in Central Europe—The significance of chemical attractants, floral morphology and concomitant insects. ACTA Societatis Botanicorum Poloniae, 80, 49-57.
- Micheneau, C., Fournel, J., Warren, H.B., Huge, S., Gauvin-Bialecki, A., Thierry, P., Dominique, S. and Chase, M.W. (2010) Orthoptera, a new order of pollinator. Annals of Botany, 105, 355-364. doi:10.1093/aob/mcp299
- Goodman, M.S. (1996) A floral and faunal inventory of the eastern slopes of the Reserve Integrale d’Andringitra, Madagascar. Fieldiana (Zoology New Series 85), 187- 188.
- FAO (1990) Area of forets and other woodland. FAO Forestry Paper Annex 1, Forest Resources Assessment, Global Synthesis, 13.
- Lorence, H.D. and Sussman, W.R. (1986) Exotic species invasion into Mauritius wet forest remnants. Journal of Tropical Ecology, 2, 147-162.
- Roberts, D.L. and Wilcock, C.C. (2001) Levels of fruiting success in the Mascarene Islands orchid flora: Implications for conservatíon. Proceedings from the 1st Orchid Conservation Congress: Incorporating the 2nd International Orchid population Biology Congress, September 2001, 78.
- Probst, J.M. (2000) L’Oiseau lunettes vert ou Zostérops vert de La Réunion Zosterops olivacea. Bulletin Phaethon, 11, 29-30.
NOTES
*Corresponding author.

