Food and Nutrition Sciences
Vol.4 No.8(2013), Article ID:34675,4 pages DOI:10.4236/fns.2013.48098
Quantitative Determination of Cd and Pb in Tissues and Organs of Chickens Raised in El-Jabel Alakhder Region—Libya
![]()
Department of Food Science and Technology, Faculty of Agriculture, Omar Almukhater University, Bayda, Libya.
Email: rabdolgader@yahoo.ca
Copyright © 2013 Ramadan E. Abdolgader et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received December 4th, 2012; revised January 8th, 2013; accepted January 17th, 2013
Keywords: Chicken; Heavy Metals; Lead; Cadmium
ABSTRACT
This study was carried out to determine the concentration of Cd, and Pb in the tissues of thigh, breast, liver, heart, gizzard, neck and skin of chicken. All samples were collected in the summer of 2004 from three different commercial farms of chickens production located in different areas of EL-Jabal Alakhder region at Libya country (Gernada, EL-Abrak and Sousa). For comparison, the metals were also determined in the same tissues of the chickens which produced in the national station of poultry production at EL-Akhoria city. The metals in the chickens feed and in water for both drinking and cleaning inside the slaughter house were also determined. The results revealed that the highest concentrations of Cd were found in neck, liver and heart while the neck and skin tissues contained the highest level of Pb. However, the tissues of thigh and breast flesh had the lowest level of metals. The levels of Cd in the different tissues ranged from 0.008 to 0.227 ppm and from 0.093 to 2.391 ppm for Pb. The results revealed that the levels of Cd in neck from all farms, liver from both EL-Akhoria and Gernada farms. Levels of Pb in the neck and skin from all farms were exceeding the permitted limits according to some European regulations. The results also indicate that the high concentrations of Cd in some tissues were due to the effect of high levels of these metals in the feed of birds. However the drinking and cleaning water had no effects on the level of the metals in the different tissues.
1. Introduction
Pollution of the environmental with heavy metals is a serious problem which is recognized in most countries of the world [1].
Trace metals have been introduced to the environmental both through natural processes and as a consequence of human activities such as industrialization or fuel combustion [2].
The toxicity of lead is attributed to the fact that it interferes with the normal function of enzymes. Biopolar lead forms strong bonds with enzymes bearing sulfhydryl groups thus inhibiting their action. Lead is toxic to the blood and the nervous, urinary, gastric and genital system. Furthermore, it is also implicated in causing carcinogenesis mutagenesis and teratogenesis in experimental animals [3].
Cadmium is considered to be one of the most toxic metals. In addition, it may accumulate in the human body and may induce kidney dysfunction, skeletal damage, reproduction deficiencies, prostate cancer, mutations, and foetal (embroyonic) death [1,4].
The presence of heavy metals in chicken meat may result from natural occurrence in the soil, from where they are taken up by the plants that feed the chicken, or due to the use of contaminated fish powder as a source of animal protein feed, or from the remnants of vehicle exhausts, which are hit by air to the source of fodder and water to drink used in poultry [5,6].
Chicken meat constitute an important part of the Libyan diet and the content of toxic metal such as lead and cadmium influences the quality of the final products, due to the very limited data on the trace element levels in the chicken raised in both private and governmental farms. The goal of this study was to determine the concentration of cadmium and lead in the tissues of thigh, breast, liver, heart, gizzard, neck and skin of commercial broiler produced in three private farms in the sites of Gernada, Al-Abrak and Sousa in El-Jabal Alakhder region Libya. For comparison, the previous metals were also determined in the same tissues of chicks produced in El-Akhoria poultry production national station. The same metals were also investigated in the feed, drinking water and cleaning water used in slaughterhouse.
2. Material and Methods
2.1. Samples
Follow up period of breeding birds in order to collect samples of this study, which included water and feed used in drinking and feeding birds and the meat and other organs of which obtained after slaughtering as will as samples of the water used during the slaughtering.
A total of 560 samples which represent of seven organs (thigh, breast, liver, heart, gizzard, neck and skin) of chickens were collected from private and governmental farms.
2.2. Reagents
Nitric acid 69% (analytical grade, BDH Ltd. Pool England) and perchloric acid 60% (analytical grade, Riedelde Haen AG Germany) were used without additional purification. Deionized water from a Milli-Q water purification (Millipore, Bedford, MA, USA) was used for the preparation of samples and standards. The element standard solutions of cadmium and lead used for calibration were prepared by diluting stock solutions of 1000 mg l L of each element supplied from BDH.
All containers and glass ware were soaked in 20% nitric acid for at least 16 hours and rinsed with distilled and deionized water before use [7].
2.3. Methods
Samples of the tissues of thigh, breast, liver, heart, gizzard, neck and skin of chicken and feed samples were homogenized separately. 2.0 ± 0.0005 g of the fresh homogenate were weighed into 150 ml pyrex beaker. 10 ml HNO3 and 3 ml 60% HClO4 were added and heated on hot plate, slowly at first, until frothing ceases. Samples were heated to white fumes of HClO4. After cooling 10 ml HCl (1 + 1) were added and transfer quantitatively to 50 ml volumetric flask [3]. For water samples, 1000 ml were evaporated until the volume became 100 ml. 15 ml HCl (37%) was added and heated on hot plate for 10 min. after cooling 15 ml HNO3 was added, the digest was again heated until the total volume was 50 ml, transfer the digested solution to 100 ml volumetric flask and brought to a volume of 100 ml with deionized water [8]. Metals ion concentration were determined as three replicates by atomic absorption spectrophotometry equipped with Slotted Tube Atomic Trap (STAT) (PU 9100X Atomic absorption spectrometer Philips) Pb was measured at 217 nm and Cd at 228.8 nm with hollow cathode lamps. The detection limit was 0.0035 mg/L for Pb and 0.005 mg/L for Cd. The recovery of metals was studied by adding known amounts of standard solution to samples. The recoveries in different materials were 89% - 95.66% for Pb and 90.55% - 94% for Cd.
All of the obtained results were converted according to the recovery percentage.
2.4. Statistical Analysis
Completely randomized design (CRD) according to [9] was used. Data were statistically analyzed and the least significant difference (LSD) was calculated at a significance level of P = 0.05.
3. Results and Discussion
3.1. Cadmium Content in Chicken Meat and Other Organs
Data presented in Table 1 indicate the mean concentration of Cd in the tissues of thigh, breast, liver, heart, gizzard, neck and skin of chicken collected from three privates farms and governmental station. All metals concentration were determined on a wet weight basis.
The Cd content in thigh samples was found to be 0.016, 0.026, 0.027, and 0.022 mg/kg in EL-Akhoria (governmental station), Gernada, Al-Abrak and Sousa (privates farms) respectively.
The highest Cd concentration was detected in Gernada and Al-Abrak farm while the lowest was detected in EL-Akoria farm.
It was surprising that Cd contents in the breast from El-Akoria was higher in the same organ from private farms, also the results from Table 1 indicate that Cd content in breast was lower than those detected in thigh.
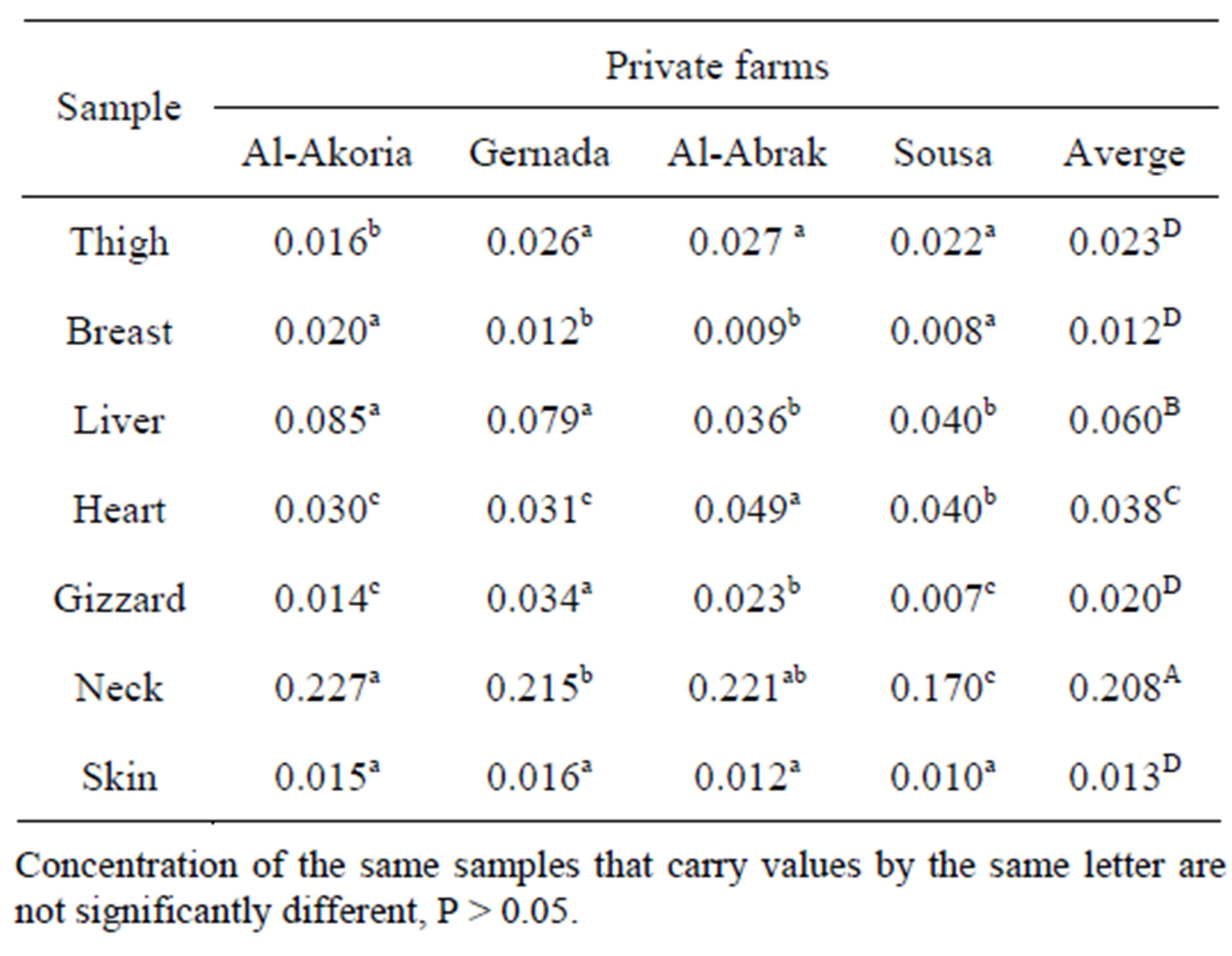
Table 1. Cd content in tissues and organs of chicken meat (mg/kg wet base).
The results obtained from Table 1 showed that the liver had relatively high Cd comparing with thigh and the highest mean concentration was detected in EL-Akoria and Gernada which was recorded as 0.085 and 0.079 mg/kg respectively on the other hand there were no significant difference between the other farms.
Maximum Cd content in heart was obtained in AlAbrak farm also as indicate in Table 1 the results showed no significant difference between Al-Akoria and Gernada from point of view of Cd content in heart.
The highest Cd content in gizzard was 0.034 mg/kg which was detected in Gernada while the lowest Cd content was found in Sousa.
The highest Cd content in all organs in this study was found in the neck which was 0.227 mg/kg obtained from El-Akhoria national station, also the other farms showed high Cd content on the other hand the skin and breast showed lower mean Cd content in all farms. The maximum Cd levels permitted for chicken samples is 0.5 mg/kg according to Saudi Arabia standards, Cd levels in chicken samples were lower than permitted levels.
Table 2 shows relationship between Cd in meat and other organs and Cd content in used fodder which means that the difference probably results from different diet. A linear relationship between Cd content in fodder and Cd content in chicken meat and other organs especially kidney and liver was reported by [10,11].
The current data show the concentration of Cd in liver and thigh were higher than those reported by [4] on the other hand similar results were reported by [12].
The mean Cd content in all farms was 0.002 mg/kg for water (Table 2) which is lower than the acceptable level in Libyan standardization for drinking water (0.005 mg/ kg).
3.2. Lead Content in Chicken Meat and Other Organs
Table 3 shows that the Pb content in the thigh samples was found to be 0.185, 0.139, 0.039, and 0.193 mg/kg in EL-Akhoria, Gernada, Al-Abrak, and Sousa respectively.
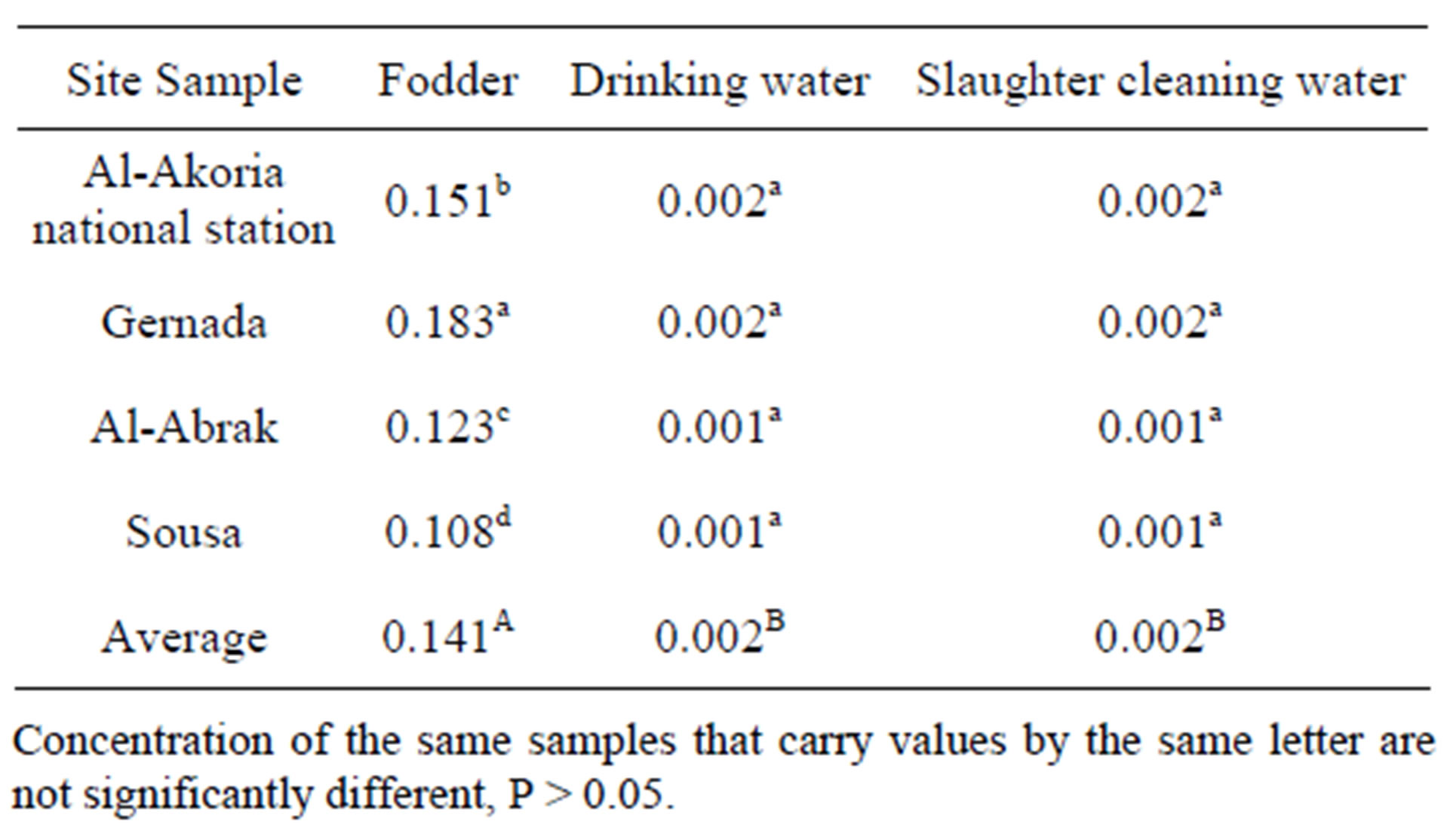
Table 2. Cd content in fodder, drinking and slaughter cleaning water (mg/kg wet base).
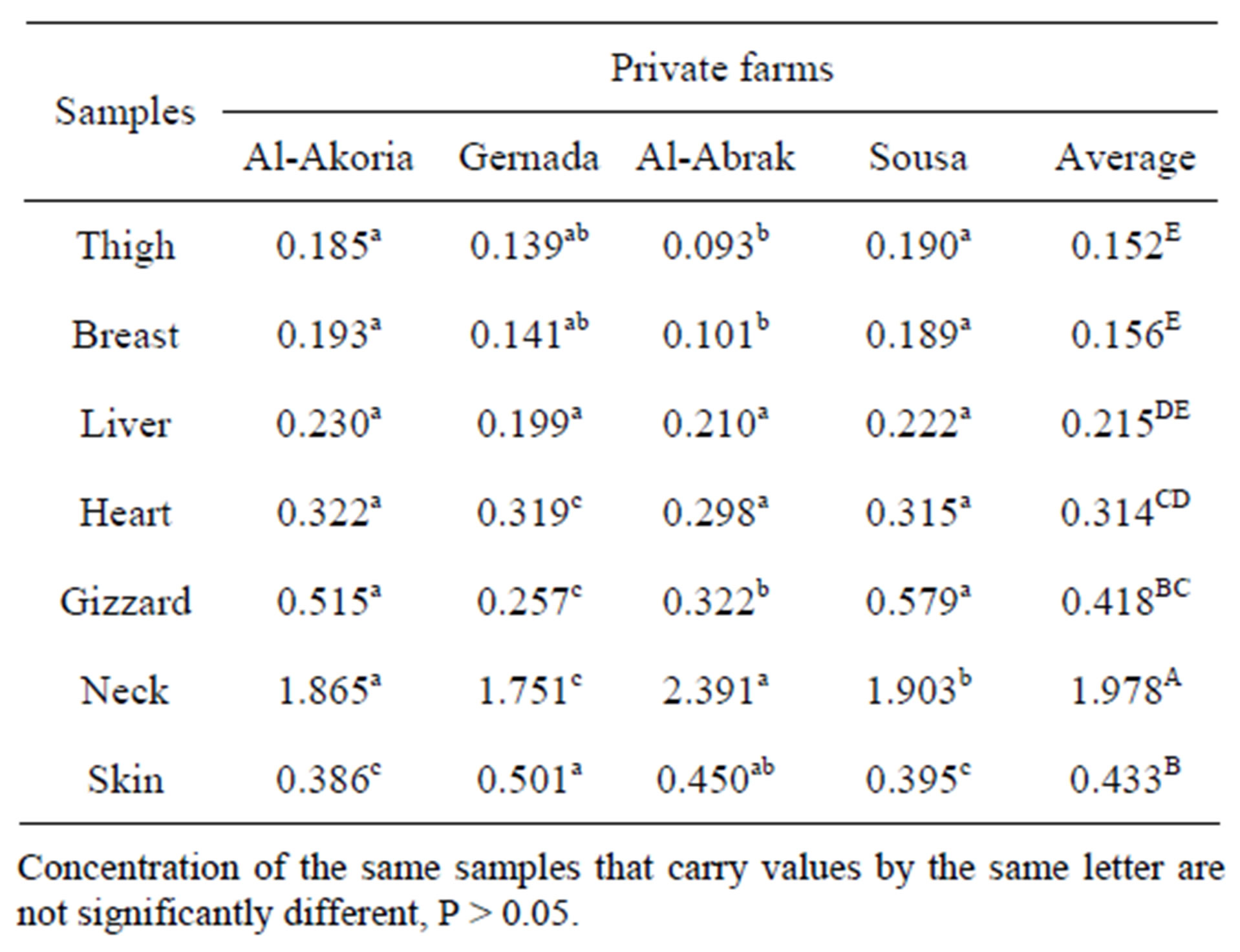
Table 3. Pb content in tissues and organs of chicken meat (mg/kg wet base).
The minimum and maximum lead content in breast were found 0.193 mg/kg in El-Akhoria and 0.101 mg/kg in Al-Abrak.
The obtained results in Table 3 shows no significant difference in Pd content in liver and heart for all farms even private or governmental farms.
Data in Table 3 proved that the highest Pd content in gizzard was detected in Sousa and El-Akhoria, which was recorded as 0.579 and 0.515 mg/kg respectively, on the other hand the lowest concentration of lead was observed in Gernada which was 0.257 mg/kg.
As well as Cd content in neck, the lead content in neck was the highest concentration comparing with the other organs, data in Table 3 indicate that the highest Pd content in neck was detected in Al-Abrak (2.391 mg/kg), EL-Akhoria (1.865 mg/kg) and Gernada (1.751 mg/kg), also the skin had higher Pb content comparing with Cd content in the same organs, and the skin ranked second after the neck from point of view lead content.
The high content of Pd and Cd in neck might be attributed to the digest of whole neck (meat tissue and bone), using whole neck in this study because some Libyan people (especially kids) in rural areas prefer to consume the whole neck.
In the literature lead level in chicken samples have been reported in the range of 0.01 - 0.40 ug/g in Turkey [4], 0.150 - 0.410 mg/kg in Egypt [13]. Lead values in chicken samples of presented work are similar to the literature values except gizzard and neck which were higher than them.
The maximum Pb levels permitted for chicken samples is 1.0 mg/kg according to Saudi Arabia standards, Pb levels in chicken samples were lower than the Saudi Arabia standards except neck which was higher than Saudi Arabia standards.
Table 4 shows lead content in fodder and drinking and
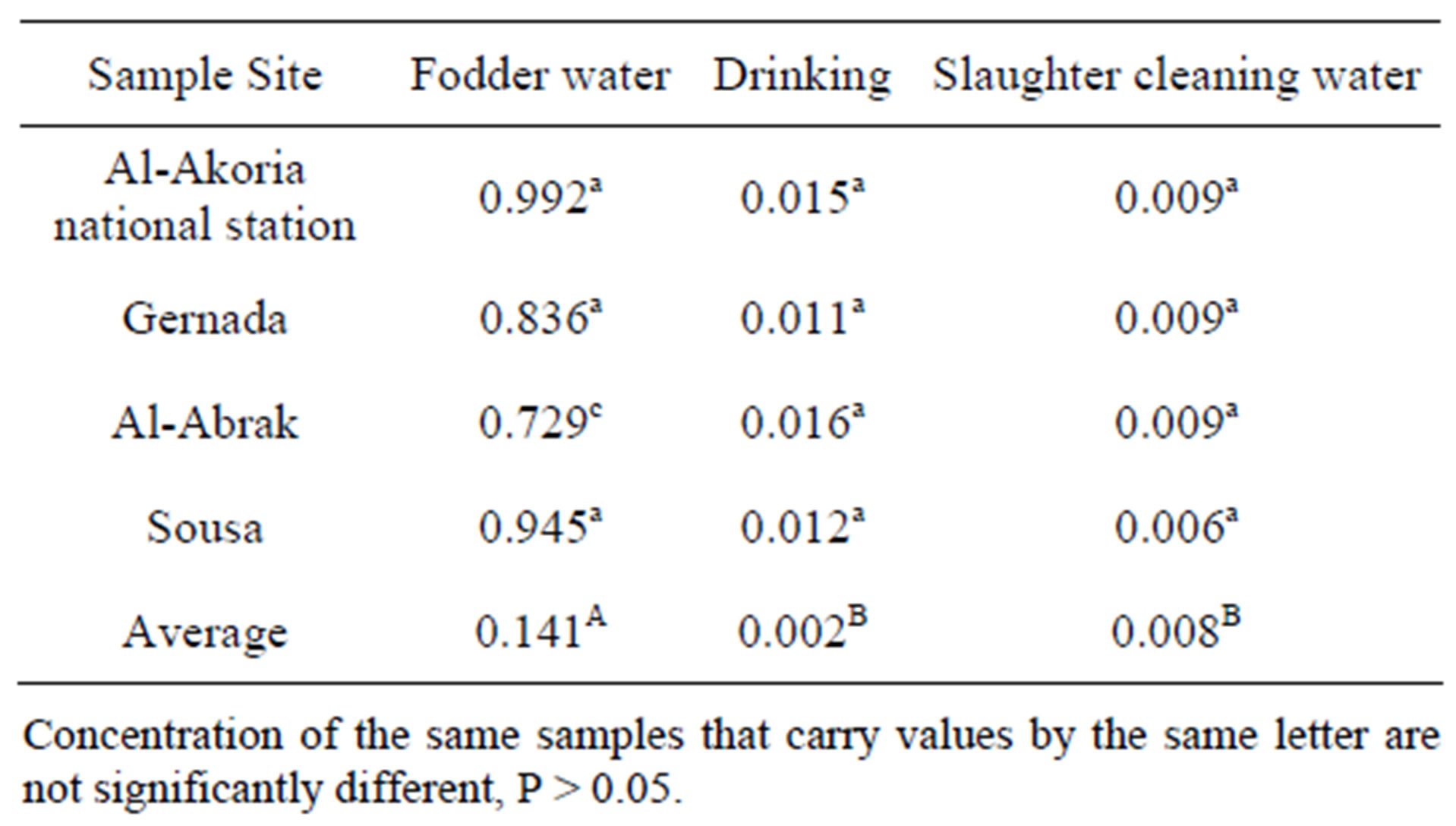
Table 4. Pb content in fodder, drinking and slaughter cleaning water (mg/kg wet base).
cleaning water, the lowest and highest Pb content in fodder were 0.729 and 0.945 mg/kg, the differences in Pb content in fodder could be attributed to using sources of fodder.
The minimum and maximum Pb content of samples were found 0.009 mg/kg in cleaning water and 0.016 mg/kg in drinking water respectively. Pb values in drinking water investigated in the presented work were found to be higher than permitted levels in Libyan standards for Pb (0.01 mg/kg).
4. Conclusion
The results indicate that the high concentrations of Cd and Pb in some tissues were due to the effect of high levels of these metals in the feed of birds. However, the drinking and cleaning water had no effects on the level of metals in different tissues.
REFERENCES
- A. A. K. Abou-arab, “Heavy Metal Contents in Egyptian Meat and the Role of Detergent Washing on Their Levels,” Food and Chemicals Toxicology, Vol. 39, No, 6, 2001, pp. 593-599. doi:10.1016/S0278-6915(00)00176-9
- M. Kosonovic, M. Y. Hasan, D. Subramaniam, A. A. F. Al-Ahbabi, O. A. Al Alkathiri, E. M. A. A. Aleassa and A. Adem, “Influence of Urbanization of the Western Coast of the United Arab Emirates on Trace Metal Content in Muscle and Liver of Wild Red-Spot Emperor (Lethrinus lentjan),” Food and Chemical Toxicology, Vol. 45, No. 11, 2007, pp. 2261-2266. doi:10.1016/j.fct.2007.06.010
- C. H. Pitot and P. Y. Dragan, “Chemical Carcinogenesis,” In: Casarett and Doull’s Toxicology, 5th Edition, Mc Graw Hill, New York, 1996, pp. 201-260.
- O. D. Uluozlu, M. Tuzen, D. Mendil and M. Soylak, “Assessment of Trace Element Contents of Chicken Products from Turkey,” Journal of Hazardous Materials, Vol. 163, No. 2-3, 2009, pp. 982-987. doi:10.1016/j.jhazmat.2008.07.050
- M. Ajmal, M. A. Khan and A. A. Domoni, “Distribution of Heavy Metals in Water and Sediments of Selected Sites of Yamuna River (India),” Environmental Monitoring and Assessment, Vol. 5, No. 2, 1985, pp. 205-214. doi:10.1007/BF00395849
- I. C. F. Damin, M. M. Silva, M. G. R. Vale and B. Welz, “Feasibility of Using Direct Determination of Cadmium and Lead in Fresh Meat by Electrothermal Atomic Absorption Spectrometry for Screening Purposes,” Spectrochimica Acta Part B: Atomic Spectroscopy, Vol. 62, No. 9, 2007, pp. 1037-1045. doi:10.1016/j.sab.2007.05.007
- “Official Methods. Method 3.2.05,” In: Official Methods of Analysis of AOAC International, 16th Edition, AOAC International, Washington DC, 1997.
- R. Salim, “Effect of Storage on the Distribution of Trace Element (Pd, Cd, Cu, Zn, and Hg) in Natural Water,” Journal of Environmental Science and Health—Part A: Environmental Science and Engineering, Vol. 22, No. 1, 1987, pp. 59-69. doi:10.1080/10934528709375333
- R. G. D. Steel and J. E. Torrie, “Principles and Procedures of Statistics: A Biometrical Approach,” Mc Graw-Hill International Book Company, New York, 1981.
- J. Gilbert, “Analysis of Food Contaminations,” Alsevier Applied Science Publishers, London, 1984.
- J. Cibulka and V. Suva, “Pd, Cd, and Hg in Liver and Muscle Tissue of Poultry in Finland,” Fakulta Agronomicka B, Vol. 45, 1986, pp. 39-50.
- A. Demirbas, “Proximate and Heavy Metal Composition in Chicken Meat and Tissue,” Food Chemistry, Vol. 67, No. 1, 1999, pp. 27-31. doi:10.1016/S0308-8146(99)00103-X
- M. H. A. Shams-Eldin and H. M. Ibrahim, “Some Heavy Metals Contents in Poultry,” J. Agric. Sci. Mansoura Univ., Vol. 15, 1990, pp. 56-61.

