Modern Research in Catalysis
Vol.06 No.02(2017), Article ID:75418,7 pages
10.4236/mrc.2017.62006
Microwave Synthesis and Photocatalytic Activity of Cadmium Indium Sulfide Nanocomposite
Kun Tu, Xiaobin Wu, Congsen Wang, Xue-Hong Liao*, Shuibin Yang*
Hubei Key Laboratory for Processing and Application of Catalytic Materials, the College of Chemical Engineering, Huanggang Normal University, Huanggang, China

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: September 12, 2016; Accepted: April 11, 2017; Published: April 14, 2017
ABSTRACT
Nanocomposite of CdIn2S4 was synthesized by direct feeding microwave synthesis method, using indium nitrate, cadmium nitrate and thioacetamide as raw material, cetyltrimethyl ammonium bromide(CTAB) as surfactant. The crystal structure, morphology and the optical property of as-prepared sample were characterized by X-ray diffraction (XRD) and scanning electron microscope (SEM) and Fluorescence spectra. The results showed that the as-prepared nanocomposite is hexagonal CdIn2S4. The SEM showed that the shape of these nanoparticles is irregular and looks like flake/sphere with some aggregation. The size of most of the aggregate is 100 to 300 nm. The photocatalytic activity of the as-prepared samples was studied by using degradation of methylene bule under visible light. The results show that the photocatalytic activity of CaIn2S4 photocatalyst was very well. When the catalyst was 1.0 g/L, C(H2O2) was 3 mL/L, after 120 min of the irradiation, the degradation rate of CdIn2S4 for methylene blue of 20 mg/L reached 86.06%.
Keywords:
Nanocomposite Cadmium Indium Sulfide, Microwave Synthesis, Photocatalysis, Visible Light, Degradation, Methylene Bule

1. Introduction
The future of modern society will depend on how we solve the urgent environmental and energy issues that we face today. Ever since the decomposition of water on a TiO2 electrode under UV light irradiation was first reported in 1972 [1] , photocatalytic processes based on semiconductors have offered the most promising solutions. They can both be used for water splitting to produce hydrogen and oxygen and for the degradation of toxic pollutants in wastewater treatment. TiO2 is by far the most widely used and investigated photocatalyst due to its high efficiency and photostability. In addition, it is non-toxic and commercially available at a low price. However, TiO2 has a major drawback that has not been overcome till date. As its band gap is rather wide (3.2 eV), TiO2 needs to be activated through UV irradiation that represents only 4% of the energy of the sunlight arriving on the earth’s surface. Therefore, the development of visible-light-driven photocatalysts has become one of the most challenging and urgent topics over the past decades. Recent progress in the field has demonstrated that the development of functionalized ternary and higher stability is an efficient strategy to overcome the intrinsic limitations of MIn2S4.
CdIn2S4 ternary sulfide because of its narrow band gap (2.1 - 2.6 ev), there is a strong absorption in the visible region, it posses unique photoelectric properties and catalytic performance [2] - [8] . Ternary metal sulfide CdIn2S4 belongs to the AB2X4 family compound semiconductor compound. The ternary semiconductor due to the narrow band gap and large specific surface area and pore structure, the sufficient contact between the catalyst and the reactant is ensured. It is conducive to the rapid migration and separation of the surface charge of the reactant can effectively inhibit the photogenerated electrons and holes of the composite, improves the photocatalytic efficiency. Binary sulfide is easy to produce light corrosion, the service life is limited, but the ternary sulfide has good stability. At present, several techniques were used to synthesize CdIn2S4, such as solventhermal (hydrothermal), high temperature decomposition, electrochemical method and so on.
In this study, we report on a direct feeding microwave synthesis method to synthesize CdIn2S4 nanophotocatalyst. The visible-light-driven photocatalytic activity of the CdIn2S4 nanophotocatalyst was evaluated with respect to methylene blue photodegradation.
2. The Experiment
2.1. Synthesis
All chemicals were analytical grade and used without further purification. CdIn2S4 materials were prepared by a direct feeding microwave synthesis method. In a typical procedure, 2.0193 g of Cd(NO3)2∙4H2O, 5.0000 g of In(NO3)3∙4.5H2O were added by stoichiometric ratio, double excess of thioacetamide and 1 g of CTAB were dissolved in 100 ml of deionized water. Then the mixed solution was placed in a microwave refluxing system to react for 20 min with a power microwave radiation of 40% and cool down naturally to the room temperature. Then the precipitate was centrifuged, washed with the deionized water for several times and dried at 60˚C in the vacuum for 4 hours. The final product was collected for the characterization.
2.2. Characterization
The crystal structure of photocatalyst was measured by XRD on a Shimadzu XRD-6100 X-ray diffractometer (Cu Kα radiation, λ = 0.15418 nm). The morphology and size of products were determined by SEM. The SEM images were recorded on a Quanta 200 FEG field emission scanning electron microscope. The optical property was obtained by Cary Eclipse fluorescence spectrometer (USA Varian Company).
2.3. Photocatalytic Experiments
The photocatalytic activities of the synthesized powders were evaluated by the degradation 100 ml of 20 mg/l of methylene bule in 0.1 g of CdIn2S4 photocatayst mixture aqueous suspension under visible-light irradiation. First, the mixed solution was placed in the dark, placed 30 minutes to reach the adsorption equilibrium. Then, in the process of photodegradation, every 10 min, the absorbance of the solution was determined by the UV-visible absorption spectrometer. The photocatalysis reaction lasted until the discoloration of the solution was fulfilled or stopped. The formula of the degradation ratio is as follow [9]
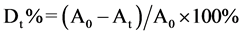 .
.
3. Results and Discussion
Figure 1 shows the XRD pattern of as-prepared sample. The XRD pattern (peak 2θ: 14.44˚, 23.62˚, 27.64˚, 28.82˚, 33.46˚, 41.12˚, 43.76˚, 47.82˚, 55.88˚, 66.48˚) showed that the product is the cubic spinel phase of CdIn2S4 (JCPDS File No. 27-0060). The diffraction peak is strong and sharp, which indicates that the sample has a high degree of crystallinity.
Figure 2 shows the SEM graph of as-prepared sample. The shape of these nanoparticles is irregular and looks like flake/sphere with some aggregation. The size of most of the aggregate is 100 to 300 nm. There are some small particles scattered around.
Figure 3 is a fluorescence spectra of as-prepared sample, the excitation wavelength is 387 nm. It can be seen that have be provided with a certain photoluminescence properties in 400 - 600 nm. The luminescence max intensity
Figure 1. X-ray diffraction pattern of as-prepared sample.
Figure 2. TEM of as-prepared sample.
Figure 3. Fluorescence spectra of as-prepared sample.
observed at 528 nm. There are relatively weak luminescence peak in 400 - 500 nm.
Figure 4 shows the typical visible absorption spectra of the photo-degradation for the methylene bule. It can be seen that the absorbance of the solution decreases rapidly along with the photocatalytic degradation reaction. After 120 min of the irradiation, the solution in the range of the visible light has almost no absorption, indicating that the methylene bule has been degraded thoroughly. Reaction of the first 10 minutes, the degradation rate is 50%. Reaction to 40 minutes, the degradation rate is 75%. Reaction to 70 minutes, the degradation rate is 80%. Reaction to 120 minutes, the degradation rate is 86.06%. When the methylene bule solution is directly put under the illumination without the catalyst, the results show that the solution does not fade by continuous illumination for 2 h, and the detection by the UV-visible absorption spectrometer finds that the absorbance does not change. CdIn2S4 nanophotocatalyst can catalyze the degradation of methylene blue solution in a short time, which shows that the photocatalytic activity of the prepared catalyst is good.
The absorbance of methylene blue solution was gradually decreased with the increase of light time, but the maximum absorption wavelength of methylene blue solution was blue shifted. Because of the conjugated structure of methylene blue, in the process of degradation, it may slowly be destroyed, resulting in the maximum absorption of methylene blue solution blue shift.
Figure 5 is the effect of the amount of the nanophotocatalyst on photocatalytic activity. As it can be seen from the figure, the most suitable amount of catalyst is 1.0 g/L. Too much or too little catalyst is unfavorable for the degradation of methyl orange solution. The amount of catalyst is too little, the use of visible light is not sufficient, the catalytic degradation rate is very slow, long time is required for the degradation. Excessive amount of catalyst leads to precipitation of the solution at the bottom results in waste and may also produce a masking effect of the incident light in the course of the reaction. The light is not sufficiently transmitted to the solution, affecting the full utilization of energy and resulting in photocatalytic efficiency on the decrease. Therefore, the right amount of catalyst is able to fully improve the photocatalytic efficiency.
Figure 4. Visible absorption spectra of photo-degradation of methylene blue.
Figure 5. Effect of dosage of the CdIn2S4 nanophotocatalyst on photocatalytic activity.
For the application of visible light catalytic degradation of organic matter, efficient visible light catalyst should not only have narrow band gap energy, should also have the right band position. The valence oxidation potential should be high enough to be able to achieve the degradation of organic matter in hole, and position of the conduction band must also be appropriate to facilitate electron reduction. The CdIn2S4 of band gap is 2.1 - 2.6 eV less than TiO2 (3.2 eV), it has a good absorption in the visible range, it is a narrow band gap photocatalyst capable of absorbing visible light. The valence band of CdIn2S4 can provide by 5s orbital of In, is also provided by the 3p6 orbit of S. When the energy is sufficient to stimulate the light, 5s of In3+ of CdIn2S4 is likely to jump to the 3d of In3+ space on the track, the energy required for this 5s electron transition is lower than that of the 3p of S electron transition. This CdIn2S4 in the ultraviolet region and visible region is formed at least two absorption bands. The absorption band is mainly formed in the visible region feature by the 5d of In3+ orbital electron transition to the 3d of Cd2+ empty orbit.
The amount of catalyst has different impact on the photocatalytic degradation of organic pollutants. When the amount of the catalyst solution is too little, the photon energy of the light source cannot be fully utilized, the reaction rate is slower, when the amount of the catalyst is excessive, the scattering catalyst light affects the transmittance of the solution, so that the reaction rate is reduced.
H2O2 plays a role in initiating the reaction of the photocatalytic degradation process. H2O2 molecule has two hydrogen atoms linked to the oxygen atom, it is positively charged more than O2. It is also easier to capture the photogenerated electrons by optical excitation, to avoid recombination of photo generated electrons and light generating holes. The addition of H2O2 promoted the efficiency of photocatalytic degradation of CdIn2S4. This may be caused by the following effects. On the one hand, H2O2 is able to capture the photogenerated conduction band electron, effectively inhibiting the recombination of electrons and holes, increase the number of highly active holes on the catalyst surface, and increase the oxidation rate of holes. On the other hand, H2O2 can generate a large number of hydroxyl radicals, therefore improve the degradation rate. However, excess H2O2 can not only improve the photocatalytic efficiency, instead, it will reduce the catalytic effect. So the amount of H2O2 added must beappropriate.
4. Conclusion
CdIn2S4 nanophotocatalyst have been prepared by the method of direct feeding microwave synthesis, this method is simple. The results showed that the as-prepared catalyst has strong photocatalytic degradation ability. When the catalyst was 1.0 g/L, C(H2O2) was 3 mL/L, the degradation rate of CdIn2S4 for methylene blue of 20 mg/L reached 86.06% in 120 min.
Cite this paper
Tu, K., Wu X.B., Wang C.S., Liao, X.-H. and Yang, S.B. (2017) Microwave Synthesis and Photocatalytic Activity of Cadmium Indium Sulfide Nanocomposite. Modern Research in Catalysis, 6, 73-79. https://doi.org/10.4236/mrc.2017.62006
References
- 1. Fujishima, A. and Honda K. (1972) Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature, 238, 37-38.
https://doi.org/10.1038/238037a0 - 2. Cai, W., Zhao, Y.S. and Hu, J. (2011) Solvothermal Synthesis and Characterization of Zinc Indium Sulfide Microspheres. Journal of Materials Science & Technology, 27, 559-562.
https://doi.org/10.1016/S1005-0302(11)60108-4 - 3. Kokate, A.V., Asabe, M.R., Delekar, S.D., et al. (2006) Photoelectrochemical Properties of Electrochemically Deposited CdIn2S4 Thin Films. Journal of Physics and Chemistry of Solids, 67, 2331-2336.
https://doi.org/10.1016/j.jpcs.2006.05.022 - 4. Kokate A.V., Asabe M.R., Delekar S.D., et al. (2007) Structural, Optical and Eletrical Studies on Pulse Electro Deposited CdIn2S4 Thin Films. Physica B: Condensed Matter, 390, 84-90.
https://doi.org/10.1016/j.physb.2006.07.069 - 5. Sawant, R.R., Rajpure, K.Y. and Bhosale, C.H. (2007) Determination of CdIn2S4 Semiconductor Parameters by (Photo) Electrochemical Technique. Physica B: Condensed Matter, 393, 249-254.
https://doi.org/10.1016/j.physb.2007.01.009 - 6. Li, Y., Dillert, R. and Bahnemann, D. (2008) Perparation of Porous CdIn2S4 Photocatalyst Films by Hydrothermal Crystal Growth at Solid/Liquid/Gas Interfaces. Thin Solid films, 516, 4988-4992.
https://doi.org/10.1016/j.tsf.2007.10.004 - 7. Zou, Z., Ye, J. and Arakawa, H. (2003) Photocatalytic Water Splitting into H2 and/or O2 under UV and Visible Light Irradiation with a Semiconductor PhotocatalystInter. International Journal of Hydrogen Energy, 28, 663-669.
https://doi.org/10.1016/S0360-3199(02)00159-3 - 8. Legrini, O., Oliveros, E. and Braun, AM. (1993) Photochemical Processes for Watertreatment. Chemical Reviews, 93, 671-698.
https://doi.org/10.1021/cr00018a003 - 9. Tang, Y., Yang, S., Zhu, Q. and Liao, X. (2016) The Microwave Synthesis and Photocatalytic Activity of W6+-Doped TiO2 Nanocomposite. Modern Research in Catalysis, 5, 45-49.
https://doi.org/10.4236/mrc.2016.52005






