Journal of Materials Science and Chemical Engineering
Vol.05 No.08(2017), Article ID:78454,11 pages
10.4236/msce.2017.58004
Ohmic Hetero-Junction of n-Type Silicon and Tungsten Trioxide for Visible-Light Sensitive Photocatalyst
Masaharu Yoshimizu1, Yuki Hotori2, Hiroshi Irie 1,2,3
1Special Doctoral Program for Green Energy Conversion Science and Technology, Interdisciplinary Graduate School of Medicine and Engineering, University of Yamanashi, Yamanashi, Japan
2Special Doctoral Program on Clean Energy, Interdisciplinary Graduate School of Medicine and Engineering, University of Yamanashi, Yamanashi, Japan
3Clean Energy Research Center, University of Yamanashi, Yamanashi, Japan

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: July 13, 2017; Accepted: August 12, 2017; Published: August 15, 2017
ABSTRACT
Visible light-sensitive photocatalyst was developed by combining n-type silicon (n-Si) and tungsten trioxide (WO3, n-Si/WO3), yielding an ohmic contact in between. In this system, the ohmic contact acted as an electron-and-hole mediator for the transfer of electrons and holes in the conduction band (CB) of WO3 and in the valence band (VB) of n-Si, respectively. Utilizing thus- constructed n-Si/WO3, the decomposition of 2-propanolto CO2 via acetone was achieved under visible light irradiation, by the contribution of holes in the VB of WO3 to decompose 2-propanol and the consumption of electrons in the CB of n-Si to reduce O2. The combination of p-type Si (p-Si) and WO3 (p-Si/ WO3), not the ohmic contact but the rectifying contact, was much less effective, compared to n-Si/WO3.
Keywords:
Ohmic Contact, Silicon, Tungsten Trioxide, Visible Light, Oxidative Decomposition, Two-Step Excitation

1. Introduction
Various photocatalytic materials have been evaluated for the oxidative decom- position of organic stains and production of hydrogen (H2) via water splitting for environmental preservation and generation of clean energy, respectively, by utilizing solar energy [1] [2] [3] . Among examined materials, titanium dioxide (TiO2) with which Fujishima and Honda first demonstrated photo induced water-splitting [1] is the most promising photocatalysts due to their high performance, abundance, nontoxicity, thermal stability and high resistance against photo-corrosion [2] [3] . Despite these advantageous properties, TiO2 is only sensitive to ultraviolet (UV) light and therefore requires modification for the utilization of visible light. To this end, numerous studies have examined the effects of doping foreign elements into TiO2 [4] and other UV-light sensitive photocatalysts, such as strontium titanate (SrTiO3) [5] , zinc oxide (ZnO) [2] [3] and so on. Another common method is to produce or find photocatalysts with narrow band-gaps which can absorb visible light [6] [7] [8] [9] . From these studies, combined systems consisting of two such narrow band-gap photocatalysts (PC1/ PC2) have been devepoled, such as tungsten disulfide (WS2)/tungsten trioxide (WO3), cobalt oxide (Co3O4)/bismuth vanadate (BiVO4), and so on (Type I in scheme 1) [10] [11] after the suggestions made in the literatures as to the more efficient charge separation in the combined system of TiO2 and cadmium sulfide (CdS), iron oxide (Fe2O3), WO3, ZnO, cupper oxide (Cu2O), or bismuth oxide (Bi2O3) etc., resulting in the increase in the lifetime of the charge carriers and the enhancement of the activity [12] [13] . However, all these combined systems are not recommended from the viewpoint of oxidation and reduction potentials of holes and electrons, respectively, after their interparticle transfer because the oxidation power of the holes and reduction powers of the electrons become weak after the transfer (Type I in Scheme 1).
To overcome the decrease in the oxidation power of the holes and reduction powers of the electrons, the insertion of a conducting layer (CL, metal such as gold (Au), silver (Ag), and tungsten (W) or reduced graphene oxide (RGO)) between two types of photocatalysts was reported [14] - [20] (PC1/CL/PC2, Type II in Scheme 1). Regarding the powdered system, CdS/Au/TiO2, WO3/W/ titanium doped-lead bismuth niobium oxide (PbBi2Nb1.9Ti0.1O9) were reported for the decomposition of organic substances [14] [15] . For the overall water-split- ting under visible light, ruthenium (Ru)-loaded rhodium-doped SrTiO3 (Ru-STO:Rh)/RGO/bismuth vanadate (BiVO4), zinc rhodium oxide (ZnRh2O4)/ Ag/silver antimonite (AgSbO3) and ZnRh2O4/Ag/bismuth vanadate (Bi4V2O11) were reported [16] - [20] . In addition, direct connection of the two or more types of photocatalysts without the conducting layer was also reported based on the
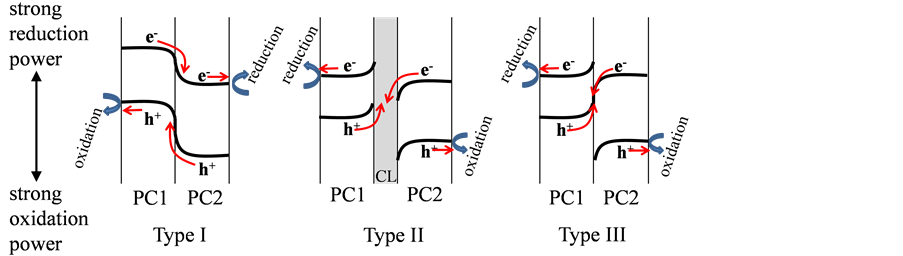
Scheme 1. Three types of previously proposed heter-junctioned photocatalysts.
concept of ohmic contact (PC1/PC2, Type III in Scheme 1). In most cases, they were a photoelectrochemical (PEC) electrode water-splitting systems, such as n-type silicon (n-Si)/Fe2O3, galium indium phosphorus (GaInP2)/galium arsenic (GaAs), three types of amorphous Si, and so on [21] [22] [23] . As for the powdered system, cupper bismuth oxide (CuBi2O4)/WO3 for the oxidative decomposition of acetaldehyde, and Ru-STO: Rh/BiVO4 and ZnRh2O4/defective AgSbO3 for the overall water-splitting were reported [17] [24] [25] . However, no experimental evidences for the formation of the ohmic contact were demonstrated in all cases but only provided the concept of the ohmic contact. Thus in the present study, we demonstrated that the formation of the ohmic contact could produce a more efficient photocatalyst than that of the rectifying contact by connecting n-Si or p-type silicon (p-Si) with WO3 (n-Si/WO3, p-Si/WO3).
2. Experimental Section
2.1. Preparations of n-Si/WO3 and p-Si/WO3 Electrodes
Single crystaln-Si(100) and p-Si(100) wafers with a thickness of 525 ± 25 µm were purchased from Kyodo International Inc. The n-Si(100) and p-Si(100) wafer surfaces were cleaned by a RCA cleaning method [26] . That is, the successive immersions of the wafers in a boiling mixture of 95% sulfuric acid (H2SO4) and 30% hydrogen peroxide (H2O2) at a volume ratio of 3:1, in a 5% hydrofluoric acid (HF) solution for 5 min, in a boiling mixture of 25% aqueous ammonium (NH3), 30% H2O2 and distilled water at a volume ratio of 1:1:3 for 15 min, again in the 5% HF solution for 5 min, and in a 40% ammonium fluoride (NH4F) solution for 5 min [26] . On the cleaned n-Si(100) or p-Si(100) surface, a WO3 film was deposited by sputtering a W metal target under oxygen (O2, 40 SCCM)/argon (Ar, 60 SCCM) gas mixture and 1.5 Pa for 16 min at substrate temperature of 400˚C, using a radio frequency (RF) magnetron sputtering apparatus (Tokuda, Model CFS-8EP). The thickness was controlled to be ~200 nm.
2.2. Preparations of n-Si/WO3 and p-Si/WO3 Powders
To obtain n-Si and p-Si powders, the purchased n-Si(100) and p-Si(100) wafers, respectively, were roughly pulverized by a mortar and then finely pulverized using a planetary ball-milling apparatus at 500 rpm for 5 min before use. Then WO3 was loaded by a liquid phase deposition (LPD) on the surface of either pulverized n-Si or p-Si powder as follows [27] . Briefly, 5.01 g of tungsten acid (H2WO4, Kanto Chemical) was dissolved in 50 mL of an aqueous solution of 2% HF. 7.45 g of boric acid (H3BO3) was dissolved in 50 mL of distilled water and was used as the reagent which acts as F− scavenger. These two solutions were mixed to use as the reaction solution for WO3 deposition. 7.72 × 10−2 g of either pulverized n-Si or p-Si powder (Si/WO3 = 1/60 wt% or 1/7.3 mol%) was stirred with the mixed solution using a magnetic stirrer for 6 h at room temperature. The reaction product was obtained by a filtration, followed by washing with sufficient distilled water and drying at 50˚C. Then the samples were heated at 500˚C for 1 h in air.
2.3. Characterizations
The crystal structures of the prepared n-Si/WO3 and p-Si/WO3 electrodes and powders were examined by X-ray diffraction (XRD) using a PW-1700 system (Panalytical). A scanning transmission electron microscope (SEM, Hitachi, S-4500) was used to observe the morphology of the prepared samples. UV-visible absorption spectra for the n-Si/WO3 and p-Si/WO3 powders were obtained by the diffuse reflection method using a V-650 (JASCO) spectrometer.
The current-voltage (I-V) analysis in the presence or absence of light from a Xe lamp (LA-251Xe, Hayashi Tokei)for the n-Si/WO3 and p-Si/WO3 electrodes were performed in a conventional two electrode system using a potentiostat at (Hokuto Denko, HSV-10). To serve an ohmic electrode, platinum was deposited on WO3 using a quick coater (Sanyu Electron Co., Ltd., SC-708) and indium (Kanto Chemical) was attached on either n-Si or p-Si.
The photocatalytic activity of the n-Si/WO3 and p-Si/WO3powdered photocatalysts were evaluated by the oxidative decomposition of gaseous 2-propanol irradiated with visible light (>420 nm, 1 mW/cm2) from the Xe lamp (the same above) equipped with a glass filter (Y-44, HOYA). For the analysis, 300 mg of the photocatalyst was uniformly spread over a 5.5-cm2 irradiation area in a 500-ml quartz vessel. Prior to the injection of 6 µmol (~300 ppm) gaseous 2-propanol, the organic pollutants (originating from the air) absorbed on the surface of the photocatalysts were first photo-oxidized into CO2 and the gas in the quartz vessel was then replaced with pure synthetic air (in the absence of CO2 and organic pollutants). Following the injection of 2-propanol, the reaction vessel was kept in the dark overnight and was then subjected to visible light irradiation to start the photocatalytic reactions. The concentrations of acetone and CO2 produced were monitored using a gas chromatograph (model GC-8A, Shimadzu Co., Ltd.).
3. Results and Discussion
3.1. Characterization of the Prepared Electrodes and Powders
Figure 1 shows XRD patterns of n-Si/WO3 and p-Si/WO3 electrodes. The faces of n-Si(100) and p-Si(100) were utilized to deposit WO3, so the peak at ~69˚ corresponding to (400) should be large, and in fact the extremely large (400) peak of n-Si was observed. However, that of p-Si was not so large, which would be attributable to the deviation from the right angle in setting p-Si/WO3 to the sample holder of the XRD apparatus. The peaks originated from WO3 on both n-Si and p-Si wafers were quite similar, and WO3 on both wafers was confirmed to have a single phase, probably the triclinic phase. WO3 in both n-Si/WO3 and p-Si/WO3 powders was confirmed to have a single phase of triclinic WO3 in the obtained XRD patterns (Figure 2). As for Si, being different from Figure 1, all the peaks originating from cubic Si were observed although some of the peaks overlapped with those from WO3. The peak intensity of Si was not as high as that
Figure 1. XRD patterns of the prepared n-Si/WO3 and p-Si/WO3 electrodes.
Figure 2. XRD patterns of the prepared n-Si/WO3 and p-Si/WO3 powders.
of WO3 because WO3 powders completely covered the surface of Si as discussed below.
Figure 3 shows the cross sectional SEM image of the n-Si/WO3 electrode. The dense WO3 film with a thickness of ~200 nm was observed, similar to p-Si/WO3 (not shown here). In Figure 4, the SEM image of the n-Si/WO3 powder is shown. The entire surface of each Si powder was covered (Figure 4(a)) by the needle-like WO3 powders (Figure 4(b)), which coincided well with the results of Deki et al. [27] . Figure 5 shows the UV-visible absorption spectra for commercially available WO3, prepared n-Si/WO3 and p-Si/WO3 powders. The absorption over a wider wavelength region (>500 nm) clearly increased for n-Si/WO3 and p-Si/WO3, indicating the successful connection of WO3 and n- or p-Si. In addition, the absorptions over 500 nm of n-Si/WO3 and p-Si/WO3 were similar within a several percent, so the amounts of WO3 connected to n-Si and p-Si were presumed to be similar.
3.2. I-V Analysis
We examined the I-V analysis in the dark and under light irradiation as shown in Figure 6. The typical rectifying I-V (typical p-n junction) behavior in p-Si/ WO3 was observed, particularly, under irradiation with light. It is plausible to consider the contact of p-Si and WO3 (n-type semiconductor). That is, p-Si has the more negative energy of Fermi level (Ef) than that of WO3 when we consider
Figure 3. A cross sectional SEM image of the n-Si/WO3 electrode.
Figure 4. SEM images of the n-Si/WO3 powder. (b) is the enlargement of (a).
Figure 5. UV-visible absorption spectra of WO3, n-Si/WO3 and p-Si/WO3 powders.
Figure 6. I-V characteristics of (a) p-Si/WO3 and (b) n-Si/WO3 heterojunctions.
the vacuum level as zero energy (Scheme 2(a)). In contrast, the ohmic I-V characteristic in n-Si/WO3 was observed. As shown in Scheme 2(b), WO3 has the more negative energy of Ef than that of n-Si, so it is probable to form the ohmic contact between n-Si and WO3. In addition, irradiated with light, the current density of n-Si/WO3 was demonstrated to be much larger than that of p-Si/WO3. This means that the interparticle charge transfer, that is, charge transfer between photo generated holes in the valence band (VB) of n-Si and photoexcited electrons in the conduction band (CB) of WO3 proceeded (Scheme 2(b)). Such an ohmic I-V characteristic was also observed in In2O3-Cu2O system with poorphotovoltaic properties [28] . However, we can anticipate that the ohmic contact will function positively in terms of photocatalytic activity as discussed below.
3.3. Decomposition of Gaseous 2-Propanol
We next examined the 2-propanol decomposition in the presence of the p-Si/ WO3 and n-Si/WO3 photocatalysts under visible-light irradiation (Figure 7). In the presence of n-Si/WO3, the evolved acetone initially increased and then decreased. This decrease was accompanied by the increase in the CO2 production. This behavior is plausible as it is known that 2-propanol decomposes into CO2, which is the final product, via acetone, the intermediate product [29] . In contrast, in the presence of p-Si/WO3, both acetone and CO2 increased monotonically up to irradiation time of ~330 h. We cannot exclude the possibility that the acetone concentration would decrease after further irradiation of visible light, accompanied by the increase in the CO2 evolution in the presence of p-Si/WO3. Even in such a case, it is readily apparent that the CO2 generation rate was smaller compared to that of n-Si/WO3 during the acetone-increasing period. In addition, the longer acetone-increasing period indicates that acetone is reluctantly decomposed to CO2 in the presence of p-Si/WO3. It is generally accepted that 2-propanol is easily decomposed to acetone; however acetone is hardly decom
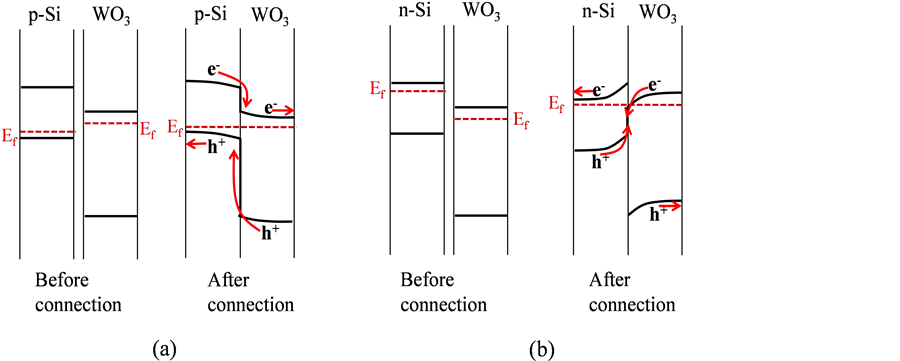
Scheme 2. Band alignments of (a) before and after connection of p-Si and WO3, and (b) that of n-Si and WO3. The charge transfer processes are also shown in the alignments after connection.
Figure 7. Changes in acetone and CO2 concentrations as functions of time in the presence of n-Si/WO3 and p-Si/WO3 under visible light irradiation.
posed to CO2. Thus, in any case, we can confidently conclude that the photocatalyticoxidative activity of n-Si/WO3 is much higher than that of p-Si/WO3.
It is well-known that the photo-produced holes play an important role in the generation of photocatalytic oxidative activity. In this sense, WO3 is a candidate for having holes with strong oxidative power because the VB top potential of WO3 is 3.1 - 3.2 V (vs. SHE, pH = 0 [30] ). The potential is even more positive than that of anatase TiO2 (3.04 V vs. SHE, pH = 0 [31] ), which has already been widely utilized as practical applications. The photo-generated electrons also play a crucial role in the generation of the photocatalytic oxidative activity. That is, to generate the photocatalytic oxidative activity, the photo-generated electrons need to be consumed in the O2 reduction because photocatalysts are usually utilized in air. If the photo-generated electrons are not consumed, the photo-produced holes will be recombined with them and eliminated. The CB bottom of TiO2 lies at −0.16 V (vs. SHE, pH = 0 [31] ), which is slightly more negative than that of one-electron O2 reduction (O2 + H+ + e− → HO2, −0.046 V vs. SHE, pH = 0 [31] ). Thus, O2 reduction is expected to proceed in the TiO2 photocatalyst. In contrast, the CB bottom of WO3 lies at 0.3 - 0.5 V vs. SHE [30] , so the photo-generated electrons cannot react with O2 through one-electron reaction. This is the reason why WO3 exhibits very low photocatalytic oxidative activity although the photo-produced holes in its VB have the strong oxidative power. To realize WO3 for the highly active photocatalyst, either Pt or Cu(II) is loaded on WO3 (Pt/WO3, Cu(II)/WO3) [32] [33] . Again, WO3 exhibits very low photocatalytic oxidative activity because the photogenerated electrons cannot reduce O2 through the one-electron reaction reduction. Pt or electron injected Cu(II) (i.e., Cu(I)) acts as a catalyst for multi-electron oxygen reduction (two electron reduction: O2 + 2H+ + 2e− → H2O2, 0.68 V; or four-electron reduction: O2 + 2H2O + 4H+ + 4 e− → 4H2O, 1.23 V [32] ).Thus the photogenerated electrons in either Pt/WO3 or Cu(II)/WO3 are consumed in the multi-electron reduction of O2.
In the n-Si/WO3 system (Scheme 2(b)), we consider that the 2-propanol decomposition performance was derived from the photo-produced holes with the strong oxidative power that were generated in the VB of WO3 contributing to 2-propanol oxidation, and the photo-excited electrons that were generated in the CB of n-Si contributing to O2 reduction through one-electron reaction. Importantly, the ohmic contact between n-Si and WO3 acts as electron-and-hole mediator for the transfer of electrons and holes in the CB of WO3 and in the VB of n-Si, respectively. Note that, to the best of our knowledge, Si (both n-Si and p-Si) does not function as the multi-electron O2 reduction catalyst. In the p-Si/ WO3 system (Scheme 2(a)), the photo-produced holes in p-Si do not have the potential to oxidize 2-propanol, considering its VB top potential. Contrastly, a portion of the photo-produced holes in WO3 that exist on its surface across the Si particle can react with 2-propanol, and the photo-excited electrons reduce WO3 itself to produce protonated WO3 (HxWO3−y) and are eliminated. Thus, p-Si/WO3 exhibited the activity for the 2-propanol decomposition, however the activity was low.
4. Conclusion
We demonstrated the ohmic-contact n-Si/WO3 system that could decompose 2-propanolinto CO2 via acetone under irradiation with visible light in comparison with the rectifying-contact p-Si/WO3 system. These results point out a promising direction for producing an efficient photocatalyst by using the ohmic direct-connection with small band-gap materials to utilize the solar spectrum more efficiently.
Cite this paper
Yoshimizu, M., Hotori, Y. and Irie, H. (2017) Ohmic Hetero-Junction of n-Type Silicon and Tungsten Trioxide for Visible-Light Sensitive Photocatalyst. Journal of Materials Science and Chemical Engineering, 5, 33-43. https://doi.org/10.4236/msce.2017.58004
References
- 1. Fujishima, A. and Honda, K. (1972) Electrochemical Photolysis of Water at a Semicon-ductor Electrode. Nature, 238, 37-38.
https://doi.org/10.1038/238037a0 - 2. Hoffmann, M.R., Martin, S.T., Choi, W. and Bahnemann, D.W. (1995) Environmental Applicdations of Semiconductor Photocatalysis. Chemical Reviews, 95, 69-96.
https://doi.org/10.1021/cr00033a004 - 3. Linsebigler, A.L., Lu, G. and Yates. Jr., T. (1995) Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chemical Reviews, 95, 735-758.
https://doi.org/10.1021/cr00035a013 - 4. Asahi, R., Morikawa, T., Ohwaki, T., Aoki, K. and Taga, Y. (2001) Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science, 293, 269-271.
https://doi.org/10.1126/science.1061051 - 5. Yoneyama, H., Koizumi, M. and Tamura, H. (1979) Photolysis of Water on Illuminated Strontium Titanium Trioxide. Bulletin of the Chemical Society of Japan, 52, 3449-3450.
https://doi.org/10.1246/bcsj.52.3449 - 6. Maeda, K., Teramura, K., Lu, D., Takata, T., Saito, N., Inoue, Y. and Domen, K. (2006) Photocatalyst Releasing Hydrogen from Water. Nature, 440, 295.
https://doi.org/10.1038/440295a - 7. Zou, Z., Ye, J., Sayama, K. and Arakawa, H. (2001) Direct Splitting of Water under Visible Light Irradiation with an Oxide Semiconductor Photocatalyst. Nature, 414, 625-627.
https://doi.org/10.1038/414625a - 8. Maruyama, Y., Irie, H. and Hashimoto, H. (2006) Visible Light Sensitive Photocatalyst, Delafossite Structuredα-AgGaO2. The Journal of Physical Chemistry B, 110, 23274-23278.
https://doi.org/10.1021/jp063406s - 9. Amao, F., Nogami, K., Abe, R. and Ohtani, B. (2008) Preparation and Characterization of Bismuth Tungstate Polycrystalline Flake-Ball Particlesfor Photocatalytic Reactions. The Journal of Physical Chemistry C, 112, 9320-9326.
https://doi.org/10.1021/jp801861r - 10. Paola, A. D., Palmisano, L. and Augugliaro, V. (2000) Photocatalytic Behavior of Mixed WO3/WS2 Powders. Catalysis Today, 58, 141-149.
https://doi.org/10.1016/S0920-5861(00)00249-2 - 11. Long, M., Cai, W., Cai, J., Zhou, B., Chai, X. and Wu, Y. (2006) Efficient Photocatalytic Degradation of Phenol over Co3O4/BiVO4 Composite under Visible Light Irradiation. The Journal of Physical Chemistry B, 110, 20211-20216.
https://doi.org/10.1021/jp063441z - 12. Bessekhouad, Y., Robert, D. and Weber, J.-V. (2005) Photocatalytic Activity of Cu2O/TiO2, Bi2O3/TiO2 and ZnMn2O4/TiO2 Heterojunctions. Catalysis Today, 101, 315-321.
https://doi.org/10.1016/j.cattod.2005.03.038 - 13. Gao, B., Ma, Y., Cao, Y., Yang, W. and Yao, J. (2006) Great Enhancement of Photocatalytic Activity of Nitrogen-Doped Titania by Coupling with Tungsten Oxide. The Journal of Physical Chemistry B, 110, 14391-14397.
https://doi.org/10.1021/jp0624606 - 14. Kim, H.G. and Jeong, E.D. (2006) Photocatalytic Ohmic Layered Nanocomposite for Efficient Utilization of Visible Light Photons. Applied Physics Letters, 89, Article ID: 064103.
https://doi.org/10.1063/1.2266237 - 15. Tada, H., Mitsui, T., Kiyonaga, T., Akita, T. and Tanaka, K. (2006) All-Solid-State Z-Scheme in CdS-Au-TiO2 Three-Component Nanojunction System. Nature Materials, 5, 782-786.
https://doi.org/10.1038/nmat1734 - 16. Iwase, A., Ng, Y.H., Ishiguro, Y., Kudo, A. and Amal, R. (2011) Reduced Graphene Oxide as a Solid-State Electron Mediator in Z-Scheme Photocatalytic Water Splitting under Visible Light. Journal of the American Chemical Society, 133, 11054- 11057.
https://doi.org/10.1021/ja203296z - 17. Kobayashi, R., Tanigawa, S., Takashima, T., Ohtani, B. and Irie, H. (2014) Silver-Inserted Heterojunction Photocatalysts for Z-Scheme Overall Pure-Water Splitting under Visi-ble-Light Irradiation. The Journal of Physical Chemistry C, 118, 22450-22456.
https://doi.org/10.1021/jp5069973 - 18. Kobayashi, R., Kurihara, K., Takashima, T., Ohtani, B. and Irie, H. (2016) A Sil-ver-Inserted Zinc Rhodium Oxide and Bismuth Vanadium Oxide Heterojunction Photo-catalyst for Overall Pure-Water Splitting under Red Light. Journal of Materials Chemistry A, 4, 3061-3067.
https://doi.org/10.1039/C5TA08468G - 19. Kobayashi, R., Takashima, T., Tanigawa, S., Takeuchi, S., Ohtani, B. and Irie, H. (2016) A Heterojunction Photocatalyst Composed of Zinc Rhodium Oxide, Single Crystal-Derived Bismuth Vanadium Oxide, and Silver for Overall Pure-Water Splitting under Visible Light up to 740 nm. Physical Chemistry Chemical Physics, 18, 27754-27760.
https://doi.org/10.1039/C6CP02903E - 20. Hara, Y., Takashima, T., Kobayashi, R., Abeyrathna, S., Ohtani, B. and Irie, H. (2017) Silver-Inserted Heterojunction Photocatalyst Consisting of Zinc Rhodium Oxide and Silver Antimony Oxide for Overall Pure-Water Splitting under Visible Light. Applied Catalysis B: Environmental, 209, 663-668.
https://doi.org/10.1016/j.apcatb.2017.03.040 - 21. Mayer, M.T., Du, C. and Wang, D. (2012) Hematite/Si Nanowire Dual-Absorber System for Photoelectrochemical Water Splitting at Low Applied Potentials. Journal of the American Chemical Society, 134, 12406-12409.
https://doi.org/10.1021/ja3051734 - 22. Khaselev, O., Bansal, A. and Turner, J.A. (2001) High-Efficiency Integrated Mul-tijunction Photovoltaic/Electrolysis Systems for Hydrogen Production. International Journal of Hydrogen Energy, 26, 127-132.
https://doi.org/10.1016/S0360-3199(00)00039-2 - 23. Reece, S.Y., Hamel, J.A., Sung, K., Jarvi, T.D., Esswein, A.J., Oijpers, J.J.H. and Nicera, D.G. (2011) Wireless Solar Water Splitting Using Silicon-Based Semiconductors and Earth-Abundant Catalysts. Science, 334, 645-648.
https://doi.org/10.1126/science.1209816 - 24. Arai, T., Yanagida, M., Konishi, Y., Iwasaki, Y., Sugihara, H. and Sayama, K. (2007) Efficient Complete Oxidation of Acetaldehyde into CO2 over CuBi2O4/WO3 Composite Photocatalyst under Visible and UV Light Irradiation. Journal of Physical Chemistry Letters, 111, 7574-7577.
https://doi.org/10.1021/jp0725533 - 25. Jia, Q., Iwase, A. and Kudo, A. (2014) BiVO4-Ru/SrTiO3: Rh Composite Z-Scheme Pho-tocatalyst for Solar Water Splitting. Chemical Science, 5, 1513-1519.
https://doi.org/10.1039/c3sc52810c - 26. Yamane, S., Kato, N., Kojima, S., Imanishi, A., Ogaea, S., Yoshida, N., Nonomura, S. and Nakato, Y. (2009) Efficient Solar Water Splitting with a Composite “n-Si/p-CuI /n-i-p a-Si/n-p GaP/RuO2” Semiconductor Electrode. The Journal of Physical Chemistry C, 113, 14575-14581.
https://doi.org/10.1021/jp904297v - 27. Deki, S., Beleke, A.B., Kotani, Y. and Mizuhata, M. (2010) Synthesis of Tungsten Oxide Thin Film by Liquid Phase Deposition. Materials Chemistry and Physics, 123, 614-619.
https://doi.org/10.1016/j.matchemphys.2010.05.024 - 28. Tanaka, H., Shimakawa, T., Miyata, T., Sato, H. and Minami, T. (2004) Electrical and Optical Properties of TCO-Cu2O Heterojunction Devices. Thin Solid Films, 469-470, 80-85.
https://doi.org/10.1016/j.tsf.2004.06.180 - 29. Irie, H., Watanabe, Y. and Hashimoto, K. (2003) Nitrogen-Concentration Dependence on Photocatalytic Activity of TiO2-xNx Powders. The Journal of Physical Chemistry B, 107, 5483-5486.
https://doi.org/10.1021/jp030133h - 30. Bamwenda, G.R., Sayama, K. and Arakawa, H. (1999) The Effect of Selected Reaction Parameters on the Photoproduction of Oxygen and Hydrogen from a WO3-Fe2+-Fe3+ Aqueous Suspension. Journal of Photochemistry and Photobiology A: Chemistry, 122, 175-183.
https://doi.org/10.1016/S1010-6030(99)00026-X - 31. Torimoto, T., Nakamura, N., Ikeda, S. and Ohtani, B. (2002) Discrimination of the Active Crystalline Phases in Anatase-Rutile Mixed Titanium(IV) Oxide Photocatalysts through Action Spectrum Analyses. Physical Chemistry Chemical Physics, 4, 5910-5914.
https://doi.org/10.1039/B207448F - 32. Irie, H., Miura, S., Kamiya, K. and Hashimoto, K. (2008) Efficient Visible Light- Sensitive Photocatalysts: Grafting Cu(II) Ions onto TiO2 and WO3 Photocatalysts. Chemical Physics Letters, 457, 202-205.
https://doi.org/10.1016/j.cplett.2008.04.006 - 33. Abe, R., Takami, H., Murakami, N. and Ohtani, B. (2008) Pristine Simple Oxides as Visible Light Driven Photocatalysts: Highly Efficient Decomposition of Organic Compounds over Platinum-Loaded Tungsten Oxide. Journal of the American Chemical Society, 130, 7780-7781.
https://doi.org/10.1021/ja800835q








