Comparative Isotherms Studies on Adsorptive Removal of Congo Red from Wastewater by Watermelon Rinds and Neem-Tree Leaves ()
1. Introduction
Water is an essential necessity for human survival whose global demand doubles every 21 years and its scarcity affects 40% of the world population (about 1.2 billion) projected to reach 2.7 billion by 2025 with water borne diseases claiming annual death rate of 5 to 10 million [1] . About 71% of the earth surface is occupied by water of which only about 0.05% is accessible for human consumption while the bulk of the remaining comprises of the inaccessible seawater, groundwater, swamps and frozen polar ice caps [2] . The scarcity of water is due to rapid population growth, increased industrialization and decreased amounts of rainfall in the previous decades [3] . More so, water pollution by untreated synthetic dye effluents released from industries has been identified as one of the consequences of worsening situation of water scarcity in the society.
Dyes are complex chemical substances that bear stable aromatic rings synthesized to impart strong and per- sistent colour that does not degrade on exposure to light [4] [5] . Although natural dyes are still in rare use, al- most all dyes in use today are synthetic with annual production of over 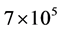 tonnes of which azo dyes ac- count for 60% - 70% [6] . About 10% - 15% of these dyes are discharged as untreated effluents during the dyeing process [7] [8] . The untreated effluent discharged from textile, cosmetics, pulp and paper, paint, pharmaceutical, food, carpet and printing industries is highly coloured due to large amounts of unfixed dyes that remained during colouring and washing [9] .
tonnes of which azo dyes ac- count for 60% - 70% [6] . About 10% - 15% of these dyes are discharged as untreated effluents during the dyeing process [7] [8] . The untreated effluent discharged from textile, cosmetics, pulp and paper, paint, pharmaceutical, food, carpet and printing industries is highly coloured due to large amounts of unfixed dyes that remained during colouring and washing [9] .
Untreated dye effluents are toxic and non-biodegradable environmental pollutants that prevent re-establish- ment of microbial populations, degrade water quality permanently, cause allergy, dermatitis, cancer, skin irrita- tion, dysfunction of kidneys, liver and reproductive system in humans [10] [11] . In other words, it could leach into and pollute surface and ground waters used for drinking; affect the photosynthesis of aquatic plants by hin- dering penetration of light into the water; and may cause suffocation of aquatic flora and fauna due to anaerobic degradation of azo dyes into highly lethal substances [12] - [14] .
Thus, to overcome the challenges of water scarcity and safe exploitation that attracted much attention from government organizations and water industries globally, it has become necessary to develop cost-effective tech- nologies for water/wastewater treatment, reclamation, recycling and reuse for sustainable industrial and agricul- tural development.
Traditional and conventional techniques usually employed for the treatment of dye wastewater consist of bio- logical, physical and chemical methods most of which are becoming inadequate due to large variability of the composition of dye wastewaters. In other words, most of these techniques are often ineffective, expensive, com- plicated, time-consuming and require highly-skilled personnel especially when the levels of dissolved dye ad- sorbates are in the range of 1 - 100 mg/L [15] . Similarly, adsorption methods using conventional adsorbents (e.g. activated carbons) poses the disadvantages of sludge disposal problems and high costs of operation, maintenance, adsorbent purchase and sludge regeneration [16] [17] .
However, proposed adsorption techniques using living and dead biomass as adsorbents are relatively cheaper, environmentally friendlier and more efficient than conventional adsorbents for the removal of dyes from waste- water even at trace level. Non-conventional adsorption method utilizes the ability of agricultural waste materials to accumulate dye pollutants from waste streams by purely physico-chemicals pathways of uptake. Their ad- sorption capacities are studied using adsorption equilibrium isotherms under such optimized conditions as agita- tion time, adsorbent dose, adsorbents particle size, initial dye concentration and initial pH of dye [17] - [19] .
Agricultural solid wastes and by-products are renewable resources available in large quantities with little or no value in most countries. Their utilization as good source of raw materials for dye removal poses the dual ad- vantages of effective wastewater treatment and waste management. They usually have high molecular weight due to the presence of lignin, cellulose and hemicelluloses components [20] . Many agricultural waste adsorbents (rice husks, corncob, coir-pith, plum kernels, bagasse pith, nut shells, fruit peels, leaf powders, spent tea leaves, fruit shells, seed husk, sawdust, hyacinth root, etc.) were reported as cost-effective alternative low cost adsorb- ents removal of dyes from wastewater in the recent past decades [21] .
2. Materials and Methods
2.1. Adsorbents Preparation
Neem tree (Azadirachta indica) leaves were collected from twigs of a number of matured tall neem trees within and near the main campus of Umaru Musa Yar’adua University, Katsina. The samples were thoroughly washed with tap-water, rinsed copiously with distilled water to remove dust and any other soluble substances. The leaves were allowed to air dry under shade at room temperature until they become crisp. The dried leaves sam- ples were then pulverized with a mechanical grinder into a powdered; and then dried overnight for 16 hours in an oven at a temperature of 65˚C. The oven-dried neem-tree leaves powder (NLP) samples were sieved to the working sizes of 75 - 300 μm using electronic sieve shaker and the fractions preserved in separately labelled air-tight plastic containers according to their particle sizes. Similar procedure was carried out on sliced pieces of fresh watermelon (Citrullus lanatus) rinds samples, collected from local fruit vendors at Kofar Kaura and Cen- tral Market in Katsina Metropolis, with the powdered fractions (WRP) separately preserved in plastic containers [21] [22] .
The analytical grade Congo red dye supplied by BDH Laboratory was used as received. Stock solution of the dye was prepared by dissolving 1 g solute in 1000 cm3 volumetric flasks to make 1000 mg/L of the dye solution [23] . Model and working calibration standards were prepared by serial dilution of the stock so- lution.
2.2. Batch Adsorption Technique
Experiments on the adsorption of Congo red by the adsorbents (WRP and NLP) were carried out by batch me- thod and the influence of various parameters such as contact time (5 - 240 min), adsorbent dosage (100 - 500 mg), particle size ( ,
,  ,
, 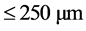 ,
, 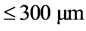 and
and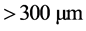 ), initial dye concentration (5 - 300 mg/L) and initial dye pH (2 - 12) were studied at constant agitation speed of 300 rpm and room temperature (25˚C) in triplicates. The adsorption measurements were conducted by mixing various amounts of adsorbent in 150 cm3 Erlenmeyer glass flasks containing 50 cm3 of dye solution of known concentration. The initial pH of the dye solutions were adjusted to the desired values by adding few drops of 0.1 M HCl or 0.1 M NaOH aqueous solutions. The solutions were agitated using orbital shaker for a predetermined time to attain equilibrium after which, the samples were removed and the supernatant solution was separated from the adsorbent by filtration using Whatman No. 41 filter paper, discarding the first few volume (3 - 4 drops) of the filtrate [24] . The filtrates were used for analyses using UV-visible spectrophotometer, reporting each data point as an average value of the triplicates recorded. In each case, the percentage adsorption and substrate’s equilibrium adsorption capacity,
), initial dye concentration (5 - 300 mg/L) and initial dye pH (2 - 12) were studied at constant agitation speed of 300 rpm and room temperature (25˚C) in triplicates. The adsorption measurements were conducted by mixing various amounts of adsorbent in 150 cm3 Erlenmeyer glass flasks containing 50 cm3 of dye solution of known concentration. The initial pH of the dye solutions were adjusted to the desired values by adding few drops of 0.1 M HCl or 0.1 M NaOH aqueous solutions. The solutions were agitated using orbital shaker for a predetermined time to attain equilibrium after which, the samples were removed and the supernatant solution was separated from the adsorbent by filtration using Whatman No. 41 filter paper, discarding the first few volume (3 - 4 drops) of the filtrate [24] . The filtrates were used for analyses using UV-visible spectrophotometer, reporting each data point as an average value of the triplicates recorded. In each case, the percentage adsorption and substrate’s equilibrium adsorption capacity, 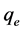 (mg/g) were evaluated using Equations (1) and (2) respectively.
(mg/g) were evaluated using Equations (1) and (2) respectively.
 (1)
(1)
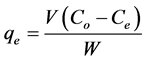 (2)
(2)
where 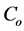 (mg/L) is the initial dye concentration,
(mg/L) is the initial dye concentration, 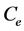 is the concentration at equilibrium or predetermined time t,
is the concentration at equilibrium or predetermined time t, 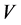 (L) is the volume of dye solution used and
(L) is the volume of dye solution used and 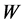 (g) is the weight of the adsorbent. The data obtained were tested against the linear forms of Langmuir, Freundlich, Temkin, Dubinin-Radushkevich (D-R) and Harkins- Jura isotherms, respectively represented as;
(g) is the weight of the adsorbent. The data obtained were tested against the linear forms of Langmuir, Freundlich, Temkin, Dubinin-Radushkevich (D-R) and Harkins- Jura isotherms, respectively represented as;
 (3)
(3)
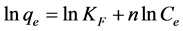 (4)
(4)
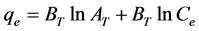 (5)
(5)
![]() (6)
(6)
![]() (7)
(7)
where Ce is any liquid phase concentration of the dye in equilibrium with the adsorbent, qe is equilibrium ad- sorption capacity of the adsorbent, qm is monolayer capacity, qD (mg/g) is the theoretical monolayer saturation capacity of adsorbent, KL is Langmuir adsorption constant, KF is Freundlich constant for relative adsorption ca- pacity of adsorbent, AT is the Temkin isotherm equilibrium binding constant (L/g), BT is the Temkins heat of adsorption, AHJ is Harkins-Jura isotherm parameter which accounts for multilayer adsorption and explains the existence of heterogeneous pore distribution, while ![]() is the isotherm constants [24] .
is the isotherm constants [24] .
3. Results and Discussion
To have an insight into the adsorption behaviours of CR dye onto watermelon rinds and neem leaves samples and to gain the optimal fitting of theoretical model, the experimental data from batch experiment were analyzed using five two-parameter isotherm equations (Langmuir, Freundlich, Dubinin-Radushkevich (D-R), Temkin and Harkins-Jura), in which linear regression analysis was used to evaluate whether the theoretical models have bet- ter or worse fit for the experimental data. The respective parameters of these isotherm models have been enu- merated in Table 1.
3.1. Langmuir Isotherm
Based on the relationship of adsorption capacity for CR dye adsorption onto the adsorbents and the equilibrium concentrations, the Langmuir adsorption isotherms are modeled and presented in Figure 1. According to these
![]()
Figure 1. Langmuir isotherm plot for CR adsorption onto WRP and NLP.
![]()
Table 1. Isotherm parameters for CR adsorption onto WRP and NLP.
isotherm curves, the Langmuir isotherm parameters are calculated and listed in Table 1.
As shown in Table 1, CR adsorption on both adsorbents have the same value of linear regression coefficient, 𝑅2 (0.9998), suggesting that the experimental data agreed closely with each other. However, the negative values of RL and KL indicates unfavourable adsorption of the dye onto the adsorbents [25] .
3.2. Freundlich Isotherm
Based on the relationship of adsorption capacity for CR dye adsorption onto the adsorbents and the equilibrium concentrations, the Freundlich adsorption isotherms are correlated and given in Figure 2, while the isotherm parameters are as presented in Table 1.
From the Table 1, CR adsorption on the adsorbents have a range of values of linear regression coefficient,R2 (0.9913 - 0.9935), demonstrating that the experimental data fitted well with the Freundlich isotherm equation, third to the Langmuir isotherm. Moreover, it was reported that the Freundlich isotherm constant can be used to explore the favourability of adsorption process. The adsorption process is said to be favourable when the value of satisfies the condition![]() , otherwise it is unfavourable. While the values in Table 1 for ad- sorption of CR on watermelon are situated outside the range of 1 - 10 indicating unfavourable adsorption proc- ess, the values for CR adsorption on neem leaves are within the range of 1 - 10, demonstrating favourable ad- sorption process [26] [27] .
, otherwise it is unfavourable. While the values in Table 1 for ad- sorption of CR on watermelon are situated outside the range of 1 - 10 indicating unfavourable adsorption proc- ess, the values for CR adsorption on neem leaves are within the range of 1 - 10, demonstrating favourable ad- sorption process [26] [27] .
3.3. Temkin Isotherm
Figure 3 illustrates the Temkin isotherm model for the dye adsorption onto the adsorbents from which the rele- vant isotherm parameters are listed in Table 1. It can be discovered in Table 1 that the values of R2 are posi- tioned within 0.9932 - 0.9947, which gave a close fit to the CR adsorption on watermelon rind and neem leaves samples, values next only to Langmuir’s model. This outcome suggests that the experimental data fitted better with the Temkin isotherm model [28] . Furthermore, it can also be discovered in Table 1 that the adsorption heat of CR adsorption on watermelon rind and neem leaves samples was restricted within −0.92 to 0.74 kJ/mol.
3.4. Dubinin-Redushkevich Isotherm
Making the linear plot according to adsorption capacity for CR dye adsorption onto the adsorbents and the equi- librium concentrations, the Dubinin-Radushkevich (D-R) adsorption isotherms (Figure 4) was obtained. Corre- sponding to which, the isotherm parameters were calculated as in Table 1.
The values of linear regression coefficient (R2) are in the range of 0.9359 - 0.9574, revealing that the experi- mental data fitted well with the Dubinin-Radushkevich (D-R) isotherm model. Moreover, it is reported that when the value of E is below 8 kJ/mol, the adsorption process can be considered as the physical adsorption. In contrast, if the value of E is located in the range of 8 - 16 kJ/mol, it is the chemical adsorption. From Table 1, it can be observed that the obtained values of mean free energy, E, are limited within the range of 0.29 - 0.32
![]()
Figure 2. Freundlich isotherm plot for CR Adsorption onto WRP and NLP.
kJ/mol. Based on these data, it can thus be concluded that the effect of physical adsorption will play a dominat- ing role in the adsorption process of CR dye adsorption onto the adsorbents [25] [29] .
3.5. Harkins-Jura Isotherm
The Harkins-Jura isotherm models for CR adsorption onto watermelon rinds and neem-tree leaves samples are presented in Figure 5 and the relevant isotherm parameters (Table 1) shows that the values of R2 are located in the range of 0.9889 - 0.9908, which indicate a better fits to the CR adsorption onto watermelon rinds and neem-
![]()
Figure 3. Temkin isotherm plot for CR adsorption onto WRP and NLP.
![]()
Figure 4. Dubinin-Redushkevich isotherm plot for CR adsorption onto WRP and NLP.
![]()
Figure 5. Harkins-Jura isotherm plot for CR adsorption onto WRP and NLP.
tree leaves samples. This result reveals that CR adsorption onto watermelon rinds and neem-tree leaves samples is in support of the multilayer adsorption rule [30] [31] .
4. Conclusion
Adsorption of CR dye onto watermelon rinds- and neem leaves-derived adsorbents has been modeled using five two-parameter isotherm equations. The results achieved suggested that all the experimental data followed the tested isotherm models and D-R, Temkin and Harkins-Jura model suggest that the dye is removed from aqueous medium by a multilayer adsorption process.