Laser Treatment of Epistaxis and Oral Bleeding in Hereditary Hemorrhagic Telangiectasia ()
1. Introduction
Hereditary hemorrhagic telangiectasia (HHT), also known as Osler-Weber-Rendu syndrome, is a relatively common, albeit under-recognized autosomal-dominant multisystemic vascular disorder. It affects all ethnic and racial groups and has a wide geographic distribution, with an overall incidence of 1 per 5000 to 10,000 persons [1] [2] [3]. The Curaçao criteria [4] for diagnosis of HHT include the following 4 criteria: Spontaneous recurrent epistaxis, multiple telangiectasias in typical locations (nose, lips, oral cavity, and skin), proven visceral arteriovenous malformations (AVM’s) (lung, liver, brain, and spine) and first-degree family member with HHT. If three or four criteria are present, a patient has “definite HHT”, while two gives “possible HHT”. De novo mutations are rare. Penetrance approaches 100% by age 40 years.
The underlying vascular angiodysplasia is responsible for the two main manifestations of the disease: telangiectasia and arteriovenous malformations [1] [2] [3]. Telangiectases are small arteriovenous shunts involving dilated arterioles and venules. They appear as red spots of 1 - 2 mm diameter on the skin. In an electron microscopy study of HHT, Braveman et al. hypothesized that the earliest clinically detectable lesion of HHT is a focal dilatation of postcapillary venules. Over time, the venules continue to enlarge and eventually connect with dilated arterioles through capillaries [5]. As the vascular lesion increases in size, the capillary segments disappear, and a direct arterio-venous communication is formed. In fully developed telangiectases, the venules are markedly dilated and convoluted, extend through the entire dermis, and have excessive layers of smooth muscle without elastic fibers. However, few reports have provided direct evidence of the actual morphogenic evolution of cutaneous telangiectatic lesions. Ultrastructural studies have so far been performed only on skin samples, and there are no available histological data on mucosal (oral and nasal) telangiectases, despite their high clinical significance. AVMs, the second most prominent lesion of HHT, lack capillaries. They consist of direct connections between arteries and veins and are larger than telangiectases. It is these vascular abnormalities that lead to the common presenting symptoms of patients with HHT.
Most telangiectases in HHT are found in the oral, nasal, and gastrointestinal mucosa and the fingertips, whereas AVMs occur most commonly in the lungs, liver, and central nervous system [6] [7]. Epistaxis due to telangiectases in the nasal mucosa is the most common and often the earliest symptom of HHT. As many as 90% of affected individuals eventually experience recurrent epistaxis, with a mean frequency of 18 episodes per month. Some patients have severe epistaxis which can lead to chronic anemia, and others have mild nosebleeds that do not require treatment. In general, the frequency and severity of the epistaxis increase with advancing age, although there are patients who report no particular change in episodes over time, and some even show improvement [8] [9].
The neoV980 is an all-purpose surgical tool for a wide scope of clinical applications. The 980 nm wavelength offering almost equal absorption in water and blood, coupled with the higher power output of the neoV platform, enables safe and effective treatment of many conditions, from ear, nose and throat (ENT) and gynecological surgery.
The neoV980 can deliver up to 20 W of output power from the fiber tip. Delivering high power energy through precision instruments and fiber optics, the unit provides sufficient power for a wide array of high value applications, enabling safe, precise, and efficient use [10].
As a result, the neoV980 can be used efficiently for precision cutting as well as coagulation of vessels for hemostasis. The aim of the present study was to describe our experience with the state-of-the-art 980 nm diode laser for the treatment of bleeding lesions of the skin, nasal and oral mucosa in patients with HHT.
2. Materials and Methods
2.1. Patients and Setting
The study group consisted of 16 patients with the diagnosis of HHT with recurrent epistaxis causing a detrimental effect on quality of life who attended a single university-affiliated, tertiary, medical center from June 2014 to October 2015. The inclusion criteria were: Patients with a diagnosis of HHT according to Curaçao criteria, Epistaxis Severity Score (ESS) [11] score over 4 (ESS 1 - 4 correlates with mild severity, 4 - 7 moderate severity and 8 - 10 is severe disease).
Exclusion criteria included: patients with an ESS score smaller than 4, patients with less than 3 criteria of the Curaçao criteria and pediatric patients.
All were treated with coagulation using the neoV 980-nm diode laser (neoLaser, Caesarea, Israel).
Hemoglobin levels and ESS scores were measured before treatment and 12 weeks after treatment.
2.2. Laser Procedure
Laser photocoagulation was performed as an office procedure under local anesthesia. For nasal lesions, anesthesia was achieved with a nasal spray of lidocaine 2% and adrenaline 1:20,000; for lesions of the skin and oral cavity mucosa, anesthesia was achieved with infiltration with lidocaine 2% and adrenaline 1:100,000. Patients were treated in continuous wave (CW) mode, with power levels starting from 12 W. The optic fiber was maintained at a distance of 3 - 5 mm from the lesion throughout. Laser shots were controlled by activating a foot pedal. Protective goggles were provided to both patient and treating physician.
The laser transmits up to 20 watts of power, either continuous or pulsed, through a flexible optical fiber consisting of a 400-micron silica core, 440-micron silica cladding with 465-micron Polyimide coating and a bare tip, measuring a length of 2.6 meters, with a numerical aperture of 0.22 at the tip. The laser may be applied with and without contact of the fiber with the tissue. The thin optical fibers are highly amenable for use inside the nasal cavity (Figure 1).
2.3. Self-Report Questionnaire
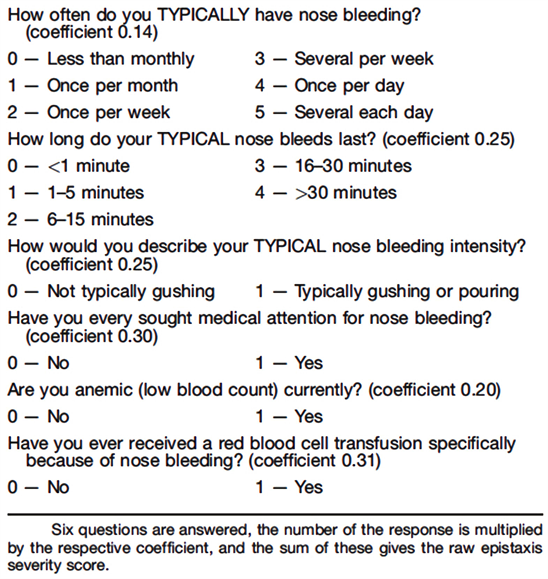
Patients were asked to complete the ESS ( [11] , see Appendix) at the clinic visits
![]()
Figure 1. The hand piece and silica fiber.
just before and after treatment. The instrument measures the severity of epistaxis in the previous month by six factors associated with self-reported severity, including: frequency, duration, intensity, need for medical attention, anemia, and need for transfusion. The normalized ESS is within a range of 0 (no epistaxis) and 10 (most severe epistaxis).
2.4. Outcome Measures
The effect of laser photocoagulation was measured by the change in hemoglobin levels and ESS scores from before to after treatment.
2.5. Statistical Analysis
Data are presented as mean ± standard deviation. The Wilcoxon signed rank sum test was used to analyze differences in the objective and subjective measure before and after treatment. All reported p values are two-sided; p < 0.05 was considered statistically significant. For the statistical analyses, we used SPSS 15.01.1 software (SPSS Inc. Chicago, IL, USA).
2.6. Ethical Considerations
All patients signed an informed consent before the laser procedure.
3. Results
Sixteen patients (10 female, 6 male) aged 29 to 76 years (average 56.6) met the inclusion criteria.
Lesion sites where the nasal mucosa in 9 patients, the nose and facial skin in 2, and the oral cavity in 5.
All patients were hemodynamically stable at the time of the laser procedure. Ten patients required one session of laser photocoagulation and 6 patients required two sessions, 2 - 6 weeks apart. The duration of follow-up ranged from 4 to 12 months.
Seven patients had a single bleeding lesion at the time of treatment (Figure 2, Figure 3). All of these patients showed complete cessation of bleeding after treatment. Their mean hemoglobin level was 8.9 ± 1.8 g/dL before treatment and 12.1 ± 1 g/dL after (p = 0.018). Corresponding mean severity of the ESS scores were 5.0 ± 1 before treatment and 0 after the treatment (p = 0.018).
Nine patients had multiple lesions at the time of treatment. In this group, we treated the most prominent lesions (1 - 4 at each session) (Figure 4, Figure 5).
Patients reported a marked improvement in the intensity of bleeding and in the frequency and severity of epistaxis, with longer bleeding-free periods. Mean hemoglobin level in this group was 8.4 ± 3.8 g/dL before treatment and 11.2 ± 1 g/dL after (in patients needing two treatment hemoglobin levels recorded after second treatment) (p = 0.008), and mean ESS severity scores were 5.33 ± 1.6 and 2.8 ± 1.1 respectively (p = 0.007).
![]()
Figure 2. Telangiectatic skin lesion at the opening of the right nostril before treatment is seen in the center.
![]()
Figure 3. The same lesion immediately after treatment.
![]()
Figure 4. Multiple teleangiectatic nasal lesions before treatment.
![]()
Figure 5. One of these lesions right after the treatment.
There were no recurrences in the treated lesions in either group during follow-up.
4. Discussion
The treatment of epistaxis consists of local measures, including application of local pressure and ice, combined with high-dose antifibrinolytic agents, such as Tranexamic acid, administered either locally, orally (1 - 2 g 3x daily) or intravenously at the time of epistaxis [12]. Systemic antiestrogen drugs such as tamoxifen have also proven very effective in most patients, but usage is limited in women of reproductive age [13]. In the presence of anemia, patients may require oral iron supplementation or, rarely, parenteral iron therapy with or without blood transfusion, depending on the level of anemia and clinical symptoms. If conservative measures are insufficient and the frequency and duration of episodes impair the patient’s quality of life, chemical and electrocoagulation may be used. However, while these modalities are tolerable as a single treatment or for a restricted area inside the nasal cavity, more extensive use poses a risk of widespread scarring and damage to the cilia and mucous glands with crust formation, leading to compromised function. Embolization of the external carotid artery branches is effective for short-term relief of symptoms, but not for long-term management. It also might lead to ischemia-related complications [14]. In very severe cases closure of the nostrils is a good treatment, but it leads to complete dysfunction of the nose [15].
Prompted by the limitations of invasive treatment, researchers have directed attention to laser photocoagulation as an alternative nonsurgical treatment modality with promising results [15] [16].
Laser ablation of nasal telangiectasias has thus gained favor in managing these patients in a safe and effective manner and offers the additional advantage of being performed on an outpatient basis. It is worthwhile to note that its benefits appear to last for only a few months, often necessitating repeated treatments. Nevertheless, it is not surprising that effective control of epistaxis episodes is associated with improved quality of life in HHT patients [17].
The most common symptom of HHT is recurrent epistaxis (up to 90% of patients), which may lead to a decrease in quality of life, hospitalizations, and the need for multiple blood transfusions [8] [9]. Oral cavity bleeding is also very common and has a significant effect on quality of life. Ideally, bleeding should be treated locally with minimum side effects and without mucosal and glandular damage. Laser photocoagulation is a potentially superior method of treatment than chemical and electrocoagulation because it avoids the risk of large areas of scar tissue and nasal stricture or vascular damage. Popular devices include the KTP laser (532 nm), the pulse dye laser (585 nm), and various diode lasers at the near infrared range (780 - 1064 nm).
The present study evaluated the effect of the neoV 980-nm diode laser. The 980 nm wavelength is selectively absorbed by hemoglobin and minimally absorbed in water, allowing for its transmission through the epithelium and coagulation of telangiectasis of the nasal mucosa, without damaging the vessel wall or mucosal-coating epithelium [18]. Because it penetrates more deeply into tissue, the neoV laser may yield better results than lower-wavelength lasers when larger vessels located a few millimeters below the mucosal surface are involved [19].
The good long-term clinical outcome of the laser-treated patients with HHT in the present study suggests the absence of a clinically significant adverse effect of the laser treatment on mucosal or glandular tissue. As the mucosa is undamaged, repeated laser sessions at minimal intervals may be used if needed without compromising nasal function.
A study by Karapantzos et al. utilizing the International Quality of Life Assessment Questionnaire corroborated these findings, indicating that laser treatment for HHT-related epistaxis produces long-lasting significant improvement in quality of life and mental health outcomes [20]. In contrast, Jørgensen et al. reported that there was no significant difference in quality of life using the SF-36 before and 6 months after laser treatment despite decreased epistaxis severity [21].
A division of the patients in our study by number of lesions affected (one vs multiple on endoscopic examination) yielded interesting findings. Patients with one lesion had an excellent outcome, with complete cessation of bleeding after the procedure and throughout follow-up (4 - 12 months). Their mean hemoglobin level increased by 3.2 g/dL, and their severity score improved by 4.0 points. These patients appear to be ideal candidates for local treatment with the 980 nm diode laser and can be spared systemic treatment with its possible side effects.
In the patients with multiple lesions, results were satisfactory. All showed a marked improvement in quality of life, with a decrease in bleeding. Mean hemoglobin increased by 2.8 g/dL and severity score improved by 2.5 points.
Limitations of the study include a small sample size and short follow-up. Sample size included all patients with retractable bleeding that met the study criteria during the study period. As for the short follow-up, the study clearly shows an improvement in symptoms and quality of life for the short term, but due to the nature of the disease, it is almost certain that these patients will have new lesions and epistaxis episode during their lifetime. The fact that the laser does not scar the mucosa significantly allows for repeat treatments if necessary.
5. Conclusion
In conclusion, the neoV 980-mm laser is an excellent treatment option for the treatment of HHT-associated epistaxis, with good results and no side effects. The procedure can be done in the office. Its use allows physicians to reserve systemic treatment for more complicated cases.
Ethics Approval and Consent to Participate
The research study was approved by the local ethics committee, a consent form detailing all the relevant information regarding the study procedures was conceived and signed by the subjects who agreed to participate.
Consent for Publication
The informed consent and agreement to participate in the study was obtained in all cases.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ Contributions
DY, UA, EY, CB, and LRW analyzed and interpreted the patient data regarding the Laser treatment of Epistaxis and Oral Bleeding in Hereditary Hemorrhagic Telangiectasia, with equal contributions in writing the manuscript. All authors read and approved the final manuscript.
Appendix—Epistaxis Severiy Score (ESS)