Nucleosides 10: Synthesis of New Derivatives of Pyrimidine and Fused Pyrimidine Nucleosides of Expected Biological Activity ()
1. Introduction
Pyrimidines and their fused derivatives form the heterocyclic core of nucleic acid bases. These ring systems are incorporated into drugs used for AIDS, cancer and anti-viral treatment. The discovery of the anti-HIV activity in AZT containing the pyrimidine nucleus has stimulated a renewed interest in these molecules and has prompted the researcher to focus work on the synthesis and study of biological properties of newer series of pyrimidine derivatives [1] [2] .
Various fused pyrimidines like purines, pteridines, quinazolines, pyridopyrimidines, triazolopyrimidines, pyrazolopyrimidines, pyrimidoazepines, fluoropyrimidines and pyrrolopyrimidines were studied in the past decade and were found to possess remarkable pharmacological properties [3] . Many pyrimidine nucleosides and analogues have been synthesized and studied for anticancer behaviour, and two are in general clinical use. A number of nucleosides were applied for remediation of breast cancer, gastrointestinal tract tumours in addition to other solid tumours [4] . Nucleosides bearing pyranosyl rings have been evaluated for their potential antiviral [5] [6] , antioxidant [7] and antibiotic [8] properties and as building blocks in nucleic acid synthesis [9] (Figure 1).
Up-to-date synthetic strategies of the acyclic and cyclic pyridinethione nucleosides including their aza- and deaza-analogs have been exploited and highlighted throughout a review in 2019 [10] .
For these biological and medicinal purposes and others, it is important to develop the field of the synthesis of nucleosides. As a part our research program dealing with the chemistry of azoles and azines nucleosides [11] - [20] , and in continuation of this work, it was considered worthwhile to study the coupling reaction of pyrimidines, and fused pyrimidines with 1-O-acetyl-2,3,5-tri-O- benzoyl-β-D-ribofuranose to get new derivatives of the corresponding nucleosides with the attempt to discover some potential compounds of medicinal importance. Furthermore, the biological activities of the new prepared nucleosides were tested using some of microorganisms.
2. Material and Methods
2.1. Chemistry
All evaporations were carried out under reduced pressure at 60˚C. TLC was carried out on aluminum sheet silica gel 60 (Fluka) and detected by UV light. All melting points were measured on an electro-thermal melting point apparatus and are uncorrected. The 1H and 13C NMR spectra were recorded in deuterochloroform (CDCl3) and deuterated dimethyl sulphoxide (DMSO-d6) at 300 MHz on a Varian Mercury VXR-300 NMR spectrometer (Cairo University) and at 600
![]()
Figure 1. Structure of commonly used nucleoside drugs.
MHz on Bruker NMR spectrometer (King Abdelaziz University). Chemical shifts were related to that of the solvent. Biological activity was carried out at the Microanalytical Center of Cairo University, Cairo, Egypt. Mass spectra were recorded on a Shimadzu GC MS-QP 1000 EX mass spectrometer at 70 eV. Elemental analyses were carried out at the Microanalytical Center of Cairo University, Giza, Egypt.
Synthesis of 6-amino-2-methylthiouracil (1b) [21] :
To a solution of 6-amino-2-thiouracil (1a) [22] (1.43 g, 10 mmol) in DMF (20 ml), potassium carbonate (1.4 g, 10 mmol) and methyl iodide (1.42 g, 10 mmol) were added and stirred at room temperature for 3 h. The reaction mixture was then diluted with cold water (20 ml) and left in refrigerator overnight. The product was filtered, dried and then recrystallized from ethanol to give colorless crystals of 1b. Yield, 1.3 g (81.9%); m.p. 261˚C - 264˚C (lit. m.p. 271˚C - 273˚C [21] ). 1H NMR (DMSO, 300 MHz), δH: 2.2 (s, 3H, SCH3), 4.9 (s, 1H, 5-CH), 6.46 (s, 2H, NH2), 11.5 (s, 1H, NH).
General Procedure for the ribosylation of 6-amino-2-thio and 2-methylthiouracils (1a) & (1b): Synthesis of 6-amino-1-(2,3,5-tri-O-benzoyl- β-D-ribofuranosyl)-2-thio and 2-methylthiouracil (4a) & (4b).
A mixture of 6-amino-2-thiouracil (1a) [22] or 6-amino-2-methylthiouracil (1b) [21] (10 mmol) and dry hexamethyldisilazane (20 ml) was heated under reflux for 24 h with a catalytic amount of ammonium sulfate. After the solution has been cooled, it was evaporated to dryness under anhydrous condition to give the silylated derivative 2a or 2b, which was directly dissolved in (15 ml) of dry 1,2-dichloroethane. To this solution was added a solution of 1-O-acetyl-2,3,5-tri- O-benzoyl-β-D-ribofuranose (3) (4.8 g, 9.6 mmol) dissolved in dry 1,2-dichloroethane (15 ml), and the mixture was treated with trimethylsilyltrifluoromethanesulfonate (2.00 ml, 10 mmol) as catalyst. After the solution had been stirred for 24 h (TLC) at room temperature, it was diluted with chloroform (30 ml), washed with a saturated solution of aqueous sodium bicarbonate (100 ml), water (3 × 20 ml) and dried over anhydrous sodium sulfate. Separation of the pure product was achieved by silica gel column chromatography with chloroform and ethyl acetate (9:1). The evaporation of the main fraction 4a or 4b, respectively was obtained as a colorless solid.
4a: Yield 2.6 g, (52%); m.p. 144˚C; 1H NMR (CDCl3, 600 MHz), δH: 4.7-4-9 (m, 4H, 5-CH, 4'H & 5'H), 5.6 - 5.6 (m, H, 3'H), 5.7 - 5.8 (m, H, 2' H), 5.9 (d, 1H, 1' H, J1',2' = 4.0 Hz), 6.6 (s, 2H, NH2), 7.26 - 8.0 (m, 15 H, 3 Ph), 10.8 (s, 1H, NH); 13C NMR (CDCl3, 150 MHz), δC: 64.1, 65.1, 72.3, 76.1,77.2, 79.4, 79.7, 95.9, 100. 5, 128.4 - 133.6, 165.2, 165.5, 166.4, 173.6 (C=S); MS: m/z = 587 (41%). Anal. calcd. for C30H25N3O8S (587.6); C, 61.32; H, 4.29; N, 7.15; S, 5.46. Found, C, 61.10; H, 4.00; N, 6.80%.
4b: Yield 2.5 g, (41%); m.p. 142˚C; 1H NMR (CDCl3, 600 MHz), δH: 2.3 (s, 3H, SCH3), 4.6 - 4.8 (m, 4H, 5-CH, 4'H & 5'H), 5.5 - 5.6 (m, H, 3'H), 5.7 - 5.8 (m, H, 2'H), 6.0 (d, 1H, 1'H, J1',2' = 4.0 Hz), 6.5 (s, 2H, NH2), 7.2 - 8.0 (m, 15H, 3 Ph), 10.5 (s, 1H, NH); 13C NMR (CDCl3, 150 MHz), δC: 15.0, 62.9, 65.0, 71.5, 72.0, 76.5, 79.9, 95.0, 101.0, 128.0 - 133.5, 165.2, 166.0, 172.5 (C=S); MS: m/z = 601 (35%). Anal. calcd. For C31H27N3O8S (601.63); C, 61.89; H, 4.52; N, 6.98; S, 5.33. Found, C, 61.10; H, 4.00; N, 6.80%.
General Procedure for the deprotection of 6-amino-1-(2,3,5-tri-O-benzoyl- β-D-ribofuranosyl)-2-thio and 2-methylthiouracil (4a) & (4b): Synthesis of 6-amino-1-(β-D-ribofuranosyl)-2-thio and 2-methylthiouracil (5a) & (5b).
A mixture of the protected nucleoside (1.0 mmol) 4a or 4b, absolute methanol (30 ml) and sodium methoxide (0.06 g, 1.1 mmol), was stirred at room temperature for 24 h (TLC). The solvent was evaporated under vacuum and the residue was dissolved in hot water and neutralized with acetic acid. The precipitate formed was filtered, dried and crystallized from water to afford 5a or 5b, respectively as colorless crystals.
5a: Yield, 135 mg, (49%); m.p259˚C; 1H NMR (DMSO-d6, 300 MHz),δH: 3.4 (m, 2H, 5', 5''H), 3.80 (m, 1H, 4'H), 4.10 - 4.2 (m, 2H, 2'H & 3'H), 4.7 (s, 1H, 5-CH), 5.0 (t, 1H, 5'-OH), 5.3 (d, J = 4.0 Hz, 1'-H), 5.5 (d, J = 4 Hz, 3'-OH), 6.1 (d, J = 4.0 Hz, 1H, 2'-OH), 8.45 (s, 1H, NH); MS: m/z = 275 (65%). Anal. calcd. For C9H13N3O5S (275.28); C, 39.27; H, 4.76; N, 15.26; S, 11.65. Found, C, 39.10; H, 5.00; N, 15.00; S, 11.5%.
5b: Yield, 160 mg, (55%); m.p 223˚C; 1H NMR (DMSO-d6, 300 MHz), δH: 2.2 (s, 3H, SCH3), 3.5 (m, 2H, 5', 5''H), 3.80 (m, 1H, 4'H), 4.0 - 4.1 (m, 2H, 2'H & 3'H), 4.8 (s, 1H, 5-CH), 5.1 (t, 1H, 5'-OH), 5.4 (d, J = 4.0 Hz, 1'-H), 5.5 (d, J = 4 Hz, 3'-OH), 6.0 (d, J = 3.8 Hz, 1H, 2'-OH); MS: m/z = 289 (45%). Anal. calcd. for C10H15N3O5S (289.31); C, 41.52; H, 5.23; N, 14.52; S, 11.08. Found, C, 41.80; H, 5.10; N, 14.40; S, 11.00%.
Ribosylation of 6-amino-1-benzyl-2-thiouracil (8): Synthesis of 6-amino- 3-(2,3,5-tri-O-benzoyl-β-D-ribofuranosyl)-1-benzyl-2-thiouracil (9).
A mixture of 6-amino-1-benzyl-2-thiouracil (8) (2.33 g, 10 mmol) and dry hexamethyldisilazane (60 ml) was heated under reflux for 24 h with a catalytic amount of ammonium sulfate. After the solution cooled, it was evaporated to dryness under anhydrous condition and the residue was dissolved in (20 ml) of dry 1,2-dichloroethane. To this solution was added a solution of l-O-acety1-2,3- 5-tri-O-benzoyl-β-D-ribofuranose (3) (4.8 g, 9.80 mmol) dissolved in dry 1,2-dichloroethane (20 ml), and the mixture was treated with (2 ml, 10 mmol) trimethylsilyl trifluoromethanesulfonate in dry 1,2-dichloroethane (10 ml) as catalyst was added dropwise. After the solution had been stirred for 24 h at room temperature (TLC), it was diluted with chloroform (20 ml), washed with a saturated solution of aqueous sodium bicarbonate (100 ml), water (3 × 20 ml) and dried over anhydrous sodium sulfate. Separation of the pure product was achieved by silica gel column chromatography with a chloroform: ethyl acetate (9:1). On evaporation of the main fraction, protected nucleoside 9 as buff crystals.
Yield 2.98 g, (40%); m.p. 179˚C; 1H NMR (CDCl3, 300 MHz), δH: 4.6 - 4.9 (m, 4H, 5-CH, 4'H & 5'H), 5.2 (s, 2H, CH2), 5.3 - 5.5 (m, 2H, 2'H & 3'H), 6.2 (d, 1H, 1'H, J1',2' = 4.0 Hz), 6.5 (s, 2H, NH2), 7.15 - 8.1 (m, 20H, 4Ph); 13C NMR (CDCl3, 75 MHz), δC: 43, 104.5, 127.0 - 132.9, 135.0, 138.1, 166.2 167.4, 173.0 (C=S); MS: m/z = 677 (M+, 20%). Anal. calcd. for C37H31N3O8S (677.72); C, 65.57; H, 4.61; N, 6.20; S, 4.73. Found, C, 65.30; H, 4.30, N, 6.10; S, 4.50%.
Deprotection of 6-amino-3-(2,3,5-tri-O-benzoyl-β-D-ribofuranosyl)-1- benzyl-2-thiouracil (9): Synthesis of 6-amino-3-(β-D-ribofuranosyl)-1-benzyl- 2-thiouracil (10).
A mixture of compound 9 (200 mg, 0.27 mmol) in absolute methanol (10 ml) and sodium methoxide (16.04 mg, 0.29 mmol) was stirred at room temperature for 24 h [TLC (chloroform/Ethyl Acetate) (9:1)]. Evaporation of the solvent under vacuum gave a white solid, which was dissolved in hot water and neutralized with acetic acid. The precipitate formed was filtered and dried to give pale yellow crystals of 10.
Yield, 100 mg, (90%); m.p 280˚C. 1H NMR (DMSO-d6, 300 MHz); δH: 4.0 - 4.2 (m, 3H, 5', 5''H & 4'H), 4.5 - 4.6 (m, 2H, 5-CH & 4'H), 5.2 - 5.5 (m, 4H, CH2, 3'H & 2'H), 5.8 - 5.9 (m, 2H, 5'-OH & 3'OH), 6.0 (m, 1H, 2'-OH), 6.3 (d, J = 4 Hz, 1H, 1'-H), 6.55 (s, 2H, NH2), 7.25 - 8.2 (m, 5H, Ph); MS: m/z = 365 (37%). Anal. calcd. for C16H19N3O5S (365.41); C, 52.59; H, 5.24; N, 11.50; S, 8.78. Found, C, 52.50; H, 5.50, N, 11.30; S, 8.60%.
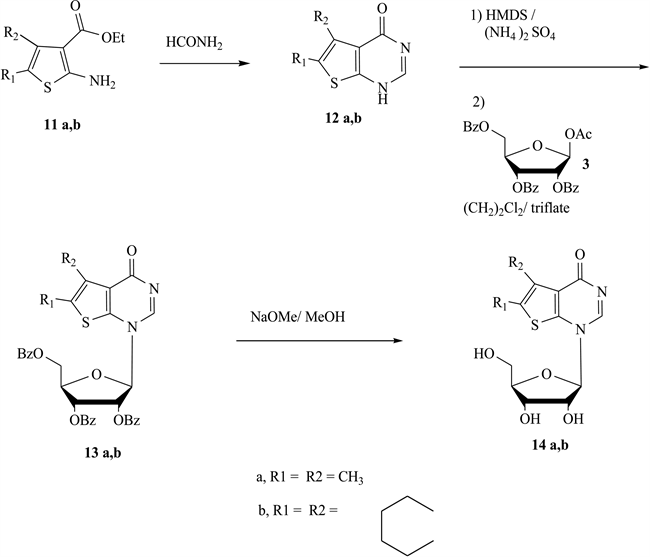
General Procedure for the ribosylation of 5,6-dimethylthieno[2, 3-d]pyrimidin-4-one (12a) and 5,6,7,8-tetrahydro-3H-benzo[4,5]thieno[2, 3-d]pyrimidin-4-one (12b): Synthesis of 1-(2,3,5-tri-O-benzoyl-β-D-ribo- furanosyl)-5,6dimethylthieno[2,3-d]pyrimidin-4-one(13a) and 1-(2,3,5-tri- O-benzoyl-β-D-ribofuranosyl)-5,6,7,8-tetrahydro-3H-benzo[4,5]thieno[2,3-d]pyrimidin-4-one (13b).
A mixture of 5,6-dimethylthieno[2,3-d]pyrimidin-4-one (12a) or 5,6,7,8-te- trahydro-3H-benzo[4,5]thieno[2,3-d]pyrimidin-4-one (12b) (10 mmol) and dry hexamethyldisilazane (30 ml) was heated under reflux 24 h with a catalytic amount of ammonium sulfate. After the solution was cooled, it was evaporated to dryness under anhydrous condition to give the silylated derivative, which was dissolved in (20 ml) of dry 1,2-dichloroethane. To this solution was added a solution of 1-O-acetyl-2,3,5-tri-O-benzoyl-β-D-ribofuranose (3) (4.6 g, 9.2 mmol) dissolved in dry 1,2-dichloroethane (10 ml) and the mixture was treated with trimethylsilyl trifluoromethanesulfonate (2.00 ml, 10 mmol) as a catalyst. After the solution had been stirred for 24 h at room temperature (TLC), it was diluted with chloroform (40 ml), washed with a saturated solution of aqueous sodium bicarbonate (100 ml) and water (3 × 20 ml) and dried over anhydrous sodium sulfate. Separation of the pure product was achieved by silica gel column chromatography with chloroform and ethyl acetate (9:1). On the evaporation of the main fraction, 13a or 13b, respectively as pale yellow crystals was obtained and recrystallized from ethanol.
13a: Yield, 2.5 g (40%); mp 111˚C; 1H NMR (CDCl3, 600 MHz),δH 2.54 (s, 3H, CH3), 2.60 (s, 3H, CH3), 4.5 - 4.9 (m, 3H, 4'H & 5'H), 5.4 (m, H, 3'H), 5.8 (m, H, 2' H), 6.4 - 6.5 (d, 1H, 1'H, J1',2' = 5.0 Hz), 7.2 - 8.2 (m, 16H, CH & 3Ph), 13C NMR (CDCl3, 150 MHz), δC: 13.5, 20.5, 63.7, 75.5, 77.3, 80.8, 86.2, 95.9, 100.1, 102.5, 123.2 - 134.1, 142.3, 157.5, 159.3, 163.3, 163.5, 165.5, 169.0. Anal. calcd. for C34H28N2O8S (624.66); C, 65.37; H, 4.52; N, 4.48; S, 5.13. Found, C, 65.60; H, 4.50; N, 4.20; S, 5.10%.
13b: Yield, 2.8 g (43%); mp 129˚C; 1H NMR (CDCl3, 600 MHz), δH 1.80 (m, 2H, CH2), 2.35 (m, 4H, 2CH2), 3.0 (t, 2H, CH2, J = 7.1 Hz), 4.25 - 4.8 (m, 3H, 4' H & 5' H), 5.20 (t, 1H, H-3', J = 5.0 Hz), 5.50 (t, 1H, H-2', J = 5.0 Hz), 6.4 (d, 1H, H-1', J1',2' = 5.1 Hz), 7.3 - 8.2 (m, 16H, CH & 3Ph); 13C NMR (CDCl3, 150 MHz), δC: 21.0, 21.5, 21.7, 22.0, 27.0, 27.5, 27.7, 29.0, 30.0, 33.0, 62.0, 68.0, 69.0, 74.1, 82.5, 120.0, 136.4, 137.0, 152.0, 158.5, 159.8, 168.0, 169.0, 169.5. Anal. calcd. for C36H30N2SO8 (650.7); C, 66.45; H, 4.65; N, 4.31; S, 4.93. Found, C, 66.30; H, 4.10; N, 4.10; S, 4.60%.
General Procedure for the deprotection of 13a and 13b: Synthesis of1-(β-D-ribofuranosyl)-5,6-dimethylthieno[2,3-d]pyrimidin-4-one (14a) and 1-(β-D-ribofuranosyl)-5,6,7,8-tetrahydro-3H-benzo[4,5]thieno[2,3-d]pyrimidin- 4-one (14b).
A mixture of the protected nucleoside 13a or 13 (2 mmol), absolute methanol (40 ml), and sodium methoxide (0.18 g, 2.1 mmol), was stirred at room temperature for 24 h (TLC). The solvent was evaporated under vacuum and the residue was dissolved in hot water and neutralized with acetic acid. The precipitate formed was filtered, dried and recrystallized from water to give pure pale yellow powder of 14a or 14b, respectively.
14a: Yield, 0.27 g (44%); mp 191˚C - 193˚C; 1H NMR (CD3OD, 600 MHz),δH 2.5 (s, 3H, CH3), 2.55 (s, 3H, CH3), 3.70 - 3.85 (m, 2H, H-5'), 4.00 (m, 1H, H-4'), 4.20 (m, 1H, H-3'), 4.35 - 4.40 (m, 1H, H-2'), 4.7 - 4.8 (t, 1H, OH-5', J = 5 Hz), 5.1 - 5.2 (d, 1H, OH-3', J = 5 Hz), 5.4 - 5.5 (d, 1H, OH-2', J = 5 Hz), 6.1 - 6.2 (d, 1H, J1',2' = 5 Hz, H-1'), 7.2 (s, 1H, CH), 13C NMR (CD3OD), δC: 14.5, 20.5, 63.0, 75.0, 77.0, 80.1, 86.1, 95.9, 100.0, 123.2 - 134.0, 142.5, 159.3, 163.5, 165.5, 170.0. Anal. calcd. for C13H16N2O5S (312.34); C, 49.99; H, 5.16; N,8.97; S, 10.27. Found, C, 49.80; H, 5.00; N, 8.90; S, 10.10%.
14b: Yield, 0.25 g, 37%; m. p.158˚C; 1H NMR (DMSO-d6, 600 MHz): δH 1.80 (m, 2H, CH2), 2.80 (m, 4H, 2CH2), 3.30 (s, 2H, CH2), 3.70 - 3.85 (m, 2H, H-5'), 4.00 (m, 1H, H-4'), 4.20 (m, 1H, H-3'), 4.35 - 4.40 (m, 1H, H-2'), 4.7 - 4.8 (t, 1H, OH-5', J = 5 Hz), 5.1 - 5.2 (d, 1H, OH-3', J = 5 Hz), 5.4 - 5.5 (d, 1H, OH-2', J = 5 Hz), 6.1 - 6.2 (d, 1H, J1',2' = 5 Hz, H-1'), 7.5 (s, 1H, CH); 13C NMR (DMSO-d6): δC 22.0, 22.0, 24.1, 25.0, 61.5, 70.5, 75.5, 85.3, 87.5, 128.0, 129.0, 130.0, 132.0, 158.0, 165.0. Anal. Calcd. for C15H18N2O5S (338.379); C, 53.24; H, 5.36; N, 8.28; S, 9.48. Found, C, 53.20; H, 5.10; N, 8.00, S, 9.30%.
2.2. Antimicrobial Assay
Cultures of two bacterial species namely, Escherichia coli EC, and Staphylococcus aureus SA as well as two fungal species, namely Aspergillus flavus AF, and Candida albicans CA were used to investigate the antimicrobial activity of ten products, namely, 4a,b; 5a,b; 9; 10; 13a,b and 14a,b. The antimicrobial activity was assayed biologically using the diffusion plate technique. The latter technique was carried out by pouring a spore suspension of the fungal species (1 cm3 of sterile water contains approximately 108 conidia) or spreading bacterial suspension over a solidified malt agar medium. The layer is allowed to set for 30 min. A solution of the test compounds ( 1.0 g = cm3) in DMSO was placed onto sterile 5 mm filter paper discs and allowed to dry, then the discs were placed on the centre of the malt agar plate and incubated at optimum incubation temperature 28˚C ± 2˚C. The bactericide Ampicillin and the fungicide Amphotericin B were used as standards under the same conditions. Measurements were considered after 72 h for fungi and 24 h for bacteria. The results are summarized in Table 1.
3. Results and Discussion
3.1. Chemistry
The first required starting materials in (Scheme 1) were 6-amino-2-thio-(1a) and 2-methylthio-(1b) uracils which were prepared according to literature reported methods [21] [22] . Ribosylation of 1a and 1b with 1-O-acetyl-2,3,5-tri-O- benzoyl-β-D-ribofuranose (3) was carried out by the silylation method according to Vorbruggen [23] by refluxing of 1a and 1b in hexamethyldisilazane (HMDS) with ammonium sulfate as a catalyst to get the silylated product 2a and 2b, respectively. Each of the silylated compounds 2a or 2b was stirred with the protected ribose 3 in dry 1,2-dichloroethane and trimethylsilane triflate; CF3SO2OSiMe3 as a catalyst at room temperature for 24 hours. This method yielded the benzoylated N-nucleosides, 6-amino-1-(2,3,5-tri-O-benzoyl-β-D-ribofuranosyl)-2-thio and 2-methylthiouracil (4a) or (4b), respectively (see Scheme 1), which hitherto have not been reported in literature yet.
The structure of the latter products (4a) or (4b) was established and confirmed on the bases of their elemental analyses and spectral data (1H & 13C NMR and MS) (see Experimental), which were consistent with the structure of nucleosides 4a and 4b. Thus, the spectral data for 4a as an example, revealed in its 1H NMR spectrum a doublet at δ = 5.9 assigned to the anomeric proton of the ribose moiety with a spin-spin coupling constant equal to 4.0 Hz that corresponds to a diaxial orientation for the 1'- and 2'-H protons, i.e., the β-configuration [1] [12] - [20] . 13C NMR spectrum of 4a revealed C-ribose moiety at chemical shifts of δ 64.1, 65.1, 72.3, 76.1, 77.2, 79.4, 79.7, 95.9, 100.5. Formation of N-nucleosides, 4a and 4b and not S-nucleosides I is based on that: 1) the formation of nucleoside (4b) is carried out from the starting material, 6-amino-2-methylthiouracil (1b), 2) 13C NMR of 4a which revealed characteristic peak at δ 173.6 for C=S group. Thus a distinction between the O-, N-, and S-glycosides was possible by comparison of 13C NMR spectra with those of literature data of similar compounds [24] [25] [26] [27] [28] . 13C C=S Chemical shifts of δ 172.0 were reported for cycloalkyl[4,5]thieno[2,3-d]pyrimidin-4-one-2-thione, while 2-alkylthiocycloalkyl- [4,5]thieno[2,3-d]pyrimidin-4-one showed chemical shifts of C-2 (C-SR) around

Scheme 1. Ribosylation of 6-amino-2-thio and 2-methylthiouracils.
δ 159. Deprotection of the protected nucleosides 4a and 4b was carried out by reaction of 4a or 4b with methanolic sodium methoxide. Hence, sirring at room temperature at 24 h (TLC) yielded the corresponding free N-nucleoside 5a or 5b, respectively (see Scheme 1). The 1H NMR of 5a showed the expected base moiety protons in addition to the sugar moiety protons, however no signal for aromatic proton appeared. However, the trial to get fused pyrimidine nucleosides, namely pyrido[2,3-d]pyrimidine nucleosides (7a,b) via the reaction of 6-amino-2-thio and 2-methylthiouracil nucleosides (4a,b) with benzylidene acetophenone (6) [29] in either ethanol or dimethylformamide according to the reported methods [30] [31] . Unfortunately it was failed (see Scheme 1).
Furthermore, 6-amino-1-benzyl-2-thiouracil nucleoside (10) was synthesized by reaction of 6-amino-1-benzyl-2-thiouracil (8)) [32] with l-O-acety1-2,3-5-tri- O-benzoyl-β-D-ribofuranose (3) to get the protected nucleoside 9, followed by debenzoylation of 9 by stirring in methanolic sodium methoxide at room temperature (Scheme 2).
In addition the ribosylation of 5,6-dimethylthieno[2,3-d]pyrimidin-4-one (11a) and 5,6,7,8-tetrahydro-3H-benzo[4,5]thieno[2,3-d] pyrimidin-4-one (11b) [33] [34] were studied by the same manner to get1-(2,3,5-tri-O-benzoyl-β-D- ribofuranosyl)-5,6-dimethylthieno[2,3-d]pyrimidin-4-one (12a) and1-(2,3,5-tri- O-benzoyl-β-D-ribofuranosyl)-5,6,7,8-tetrahydro-3H-benzo [4,5]thieno-[2,3-d]- pyrimidin-4-one (12b), respectively. Debenzoylation of each of 12a and 12b in methanolic sodium methoxide at room temperature afforded 1-(β-D-ribofuranosyl)- 5,6-dimethylthieno[2,3-d] pyrimidin-4-one (13a) and 1-(β-D-ribofuranosyl)- 5,6,7,8-tetrahydro-3H-benzo[4,5]thieno[2,3-d] pyrimidin-4-one (13b) (Scheme 3).
3.2. Antimicrobial Activity
Ten products, namely, 4a,b; 5a,b; 9; 10; 13a,b and 14a,b were evaluated for their antibacterial and antifungal activities in vitro against gram negative bacteria [Escherichia coli (EC) and gram positive bacteria [Staphylococcus aureus (SA)] and against fungal, Aspergillus flavus (AF) and Candida albicans (CA). The antibacterial and antifungal activities were carried out in the Microbiology Division of Microanalytical Center of Cairo University, using the diffusion plate

Scheme 2. Synthesis of 6-amino-1-benzyl-2-thiouracil nucleoside.
Scheme 3. Synthesis of thieno[2,3-d]pyrimidin-4-one nucleosides.
method [35] [36] , a bottomless cylinder containing a measured quantity (1 mI, mg/mL) of the sample is placed on ( 9 cm diameter) containing a solid bacterial medium (nutrient agar broth) or fungal medium (Dox`s medium) which has been heavily seeded with the spore suspension of the test organism. After incubation (24 h for bacteria and 5 days for fungi), the diameter of the clear zone of inhibition surrounding the sample is taken as measure of the inhibitory power of the sample against the particular test organism. The reference antibiotics Ampicillin (antibacterial agent) and Amphotericin B (antifungal agent) were used as references to evaluate the potency of the tested compounds under the same condition. The test results are depicted in Table 1 on the following basis: The solvent used was dimethylsulfoxide and concentration of the sample in 100 µg/ml.
The test results revealed that the compounds, 5a,b; 10; 13b and 14b exhibited moderate activity against the two bacteria species and all compounds except 14b showed no activity against the two fungi species (Table 1).
4. Conclusion
In conclusion, new selective N-nucleosides and not S-nucleosides were synthesized by using silylation method in good yields. Such nucleosides were of 2-thio and 2-methylthiouracil and 1-benzyl-2-thiouracil as pyrimidine derivatives as
![]()
Table 1. Antibacterial and antifungal activities of some of the synthesized compounds.
Inhibition Zone Diameter (IZD*) (mm/mg Compound Tested). *IZD = 2 - 9 mm beyond control = (low activity). IZD = 10 - 20 mm beyond control = (moderate activity). IZD = 20 - 30 mm beyond control = (high activity).
well as 5,6-dimethyl-thieno[2,3-d]pyrimidin-4-one and 5,6,7,8-tetrahydro-3H- benzo-[4,5]thieno-[2,3-d]pyrimidine-4-one as fused pyrimidine derivatives. The new nucleosides formed were structurally characterized and tested for biological activity against fungi and bacteria species. Some of the tested products showed moderate activity and the results were reported. Further exploration of the synthesis of N and/or S-nucleosides and their investigations to get antiviral agents are currently under studying in our laboratory.