Simple HPLC Analysis of Hinokitiol in Skin Lotion with Visible Light Detection after Pre-Column Dabsylation ()
1. Introduction
Hinokitol (Figure 1, β-thujaplicin, 4-isopropyl-2-hydroxycyclopenta-2,4,6-trien- 1-one) is a naturally occurring toxic tropolone, containing an unsaturated seven-membered carbon ring. The compound is found in heartwood of several cupressaceous plants, such as western red cedar (Thujaplicata), eastern white cedar (Thuja occidentalis), hinoki cypress (Chamaecypraisobtusa), and hiba (Thujopsis dolabrata) [1] [2] . It has a potent anti-bacterial activity (minimum inhibitory concentration of 0.2 μg/mL for Staphylococcus epidermis and Daedalea dickinsii) [2] [3] , and is used as an additive in various cosmetic products, including skin lotion and body soap.
GC and HPLC analyses of hinokitiol are not straightforward, because the tropolone ring has chelating ability, is relatively unstable and is adsorbed on the stationary phase. However, quantitative determinations of hinokitiol by GC and capillary GC have been performed after derivatization with trimethylsilyl chloride and with diazomethane, respectively [1] [4] . Hanafusa et al. [5] determined hinokitiol in cosmetics by HPLC with UV detection following formation of a hinokitiol-copper (II) complex by addition of copper (II) to the mobile phase, but they did not describe the sensitivity of the method. Also, waste containing copper (II) is toxic. Endo et al. [6] reported a sensitive HPLC determination of hinokitiol after difluoroborane derivatization; the detection limit was 40 pg of hinokitiol. Dyrskov et al. [7] determined hinokitiol by capillary zone electrophoresis, which gave a detection limit of 0.21 μM. An HPLC-dual UV (240 and 345 nm) method of hinokitiol determination in personal care products was established using a reversed-phase C4 column, providing detection limits of 0.005 μg/mL (absolute amount of 1 ng) and 0.01 μg/mL (absolute amount of 2 ng) at 240 and 345 nm, respectively [8] . Also, a simple determination method of hinokitiol in a skin lotion was recently established by HPLC-UV using 4-fluoro-7- nitro-2,1,3-benzoxadiazole(NBD-F) as a pre-column UV-labeling agent [9] . How- ever, parabens (general preservatives often added to cosmetics) or their degradation products generated interfering peaks, so the method was only appropriate for hinokitiol analysis in paraben-free samples. In addition, the labeling reagent is expensive, although the method is sensitive (detection limit: 0.33 ng). More
![]()
Figure 1. Scheme of hinokitiol derivatization with Dabsyl-Cl.
seriously, hinokitiol determination in tested cosmetics showed poor reproducibility [8] [9] .
The aim of the present study was to develop a simple, sensitive and economical assay of hinokitiol that would be suitable for quality assessment of personal care products containing parabens or their degradation products. We focused on 4-(dimethylamino)azobenzene-4’-sulfonyl chloride (Dabsyl-Cl) as a visible light (VIS) labelling agent for primary or secondary amino and phenolic hydroxyl groups [10] [11] . The sulfonyl chloride group of Dabsyl-Cl reacts with the hydroxyl group of hinokitiol (pKa 7.29 at 25˚C [6] ) in alkaline media as shown in Figure 1. Here, we describe an HPLC assay of hinokitiol in personal care products with VIS detection after pre-column derivatization with Dabsyl-Cl. We confirmed that there was little interference from paraben-related peaks.
2. Materials and Methods
2.1. Materials
Hinokitiol, Dabsyl-Cl, methyl 4-hydroxybenzoate, ethyl 4-hydroxybenzoate, propyl 4-hydroxybenzoate, isopropyl 4-hydroxybenzoate, butyl 4-hydroxyben- zoate, isobutyl 4-hydroxybenzoateand benzyl 4-hydroxybenzoate were obtained from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). General reagents were obtained from Wako Pure Chemical Industries (Osaka, Japan). Skin lotions A and B were purchased from a market in Kanazawa city, Ishikawa Pref., Japan. Skin lotion A contained hinokitiol at 0.05 g/100mL (labeled concentration). Skin lotion B also contained hinokitiol, but the level was not stated on the label.
2.2. Chromatographic Conditions
The HPLC system consisted of a model LC-10ATvp pump (Shimadzu, Kyoto, Japan), a Rheodyne injection valve (Cotati, CA, U.S.A.) with a 20-μL loop, and a model SPD-10Avp UV/VIS detector (Shimadzu) operating at 450 nm. The HPLC column (C18-MS-II, Nacalaitesque, Kyoto, Japan) was 150 mm × 3.0 mm i.d., containing 5 μm particles of C18 packing material. Quantification of peaks was performed using a Chromatopac Model C-R8A integrator (Shimadzu). The mobile phase was prepared by addition of acetonitrile (720 mL) to 280 mL of Milli-Q water containing trifluoroacetic acid (0.1 v/v%). The samples were eluted from the column at room temperature at a flow rate of 0.40 mL/min.
2.3. Preparation of Standard Solutions
A stock solution of hinokitiol (400 μg/mL) in 5% ethanol was prepared in dark glass bottle and stored at 4˚C. It was diluted with water to prepare working solutions of 0, 1.25, 2.5, 5, 10, 20 and 40 μg/mL.
2.4. Derivatization
Ultrapure water was obtained from a Milli-Q water purification system (Simplicity® UV, Millipore Corporation, Bedford, MA, U.S.A.). Borate buffer (0.1 M) was adjusted to pH 9.5 by addition of NaOH. Borate buffer (50 μL) was added to each working standard solution (50 μL), and then saturated Dabsyl-Cl solution in acetonitrile (supernatant after centrifugation of 1 mg/mL suspension, 300 μL) was added. The mixture was vortexed and allowed to react for 10 min at 55˚C. Ice-cold saturated L-aspartate solution (supernatant after centrifugation of 5 mg/mL of L-aspartate suspension, 300 μL) was added to stop the reaction. Then, an aliquot (20 μL) of the solution was injected into the HPLC system.
2.5. Application to Skin Lotion Samples and Addition-Recovery Tests
Test skin lotion A (0.25 mL) and test skin lotion B (1.0 mL) were each diluted to 20 mL with water. These diluted samples were analyzed after derivatization as described above. Addition-recovery tests were carried out to assess the accuracy of the method by spiking each skin lotion with hinokitiol (100 μg, 250 μL of stock solution), and the hinokitiol concentration in an aliquot of 50 μL was determined. Recovery was calculated as follows:
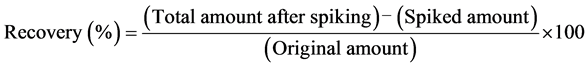
3. Results and Discussion
3.1. Derivatization of Hinokitiol with Dabsyl-Cl
For the time-course study, the reaction time was set at 1, 3, 5, 10 and 15 min at 55˚C. Hinokitiol (50 μL, 40 μg/mL), borate buffer (50 μL, pH 9.5) and saturated Dabsyl-Cl solution in acetonitrile (300 μL) were mixed as described in Materials and Methods. The derivatization of hinokitiol reached a plateau at 5 min (more than 98% of the maximal peak area), and the peak area was maximalat 10 min (Figure 2). The peak area remained stable until 15 min.
Temperature dependency was tested for the derivatization time of 5 min, as shown in Figure 3. Reaction temperature was set at 50˚C, 55˚C, 60˚C, and 65˚C. The peak area of Dabsyl-hinokitiol was stable at 50˚C to 60˚C, but decreased somewhat at 65˚C.
pH dependency (pH 7.5 to 10.0) was examined for the derivatization time of 5 min at 55˚C. Peak area of Dabsyl-hinokitiol was maximal at pH 9.5 (Figure 4). Under pH 8.5, the peak area of the derivative was gradually decreased.
When half-saturated Dabsyl-Cl solution in acetonitrile was utilized under optimal conditions, the peak area was reduced to about 50% (data not shown).
Thus, we selected the derivatization time of 10 min at 55˚C and pH 9.5 with saturated Dabsyl-Cl solution in acetonitrile for the assay.
3.2. Chromatogram
Figure 5 shows typical chromatograms obtained from (a) blank and (b) standard sample (10 μg/mL). The retention time of Dabsyl-hinokitiol was 6.8 min. The running time was 15 min.
![]()
Figure 2. Time course of formation of the Dabsyl derivative of hinokitiol. Standard sample (40 μg/mL) was reacted with Dabsyl-Cl in borate buffer, pH 9.5, at 55˚C. Data are expressed as mean values of two experiments.
![]()
Figure 3. Temperature dependency of formation of the Dabsyl derivative of hinokitiol. Standard sample (40 μg/mL) was reacted with Dabsyl-Cl for 10 min in borate buffer at pH 9.5. Data are expressed as mean values of two experiments.
![]()
Figure 4. pH dependency of formation of the Dabsyl derivative of hinokitiol. Standard sample (40 μg/mL) was reacted with Dabsyl-Cl for 10 min at 55˚C in borate buffer at various pH values. Data are expressed as mean values of two experiments.
![]()
Figure 5. Typical chromatograms of blank (a) and standard sample ((b), 10 μg/mL) after derivatization with Dabsyl-Cl. Samples were reacted with Dabsyl-Cl for 10 min in borate buffer, pH 9.5, at 55˚C. Retention time of Dabsyl-hinokitiol derivative: 6.8 min (arrowed peak).
3.3. Standard Curves of Hinokitiol
A standard curve was constructed by plotting integrated peak area vs. concentration of hinokitiol. The plot was linear (y = 33.95x + 3.03) in the range of 1.25 to 40 μg/mL with an r2 value of 0.9991. The values of the lower limits of quantification and detection were 0.60 μg/mL (absolute amount of 0.86 ng/20μL injection, signal-to-noise ratio of 10:1) and 0.18 μg/mL (absolute amount of 0.26 ng/ 20μL injection, signal-to-noise ratio of 3:1), respectively. As shown in Table 1, the sensitivity (absolute amount) of the presented method can be classed as moderate, compared with previously reported methods [6] [7] [8] [9] . However, it was about 1.3- to 7.7-fold better than that of our previous method [8] [9] .
3.4. Precision and Accuracy
Precision and accuracy for intra-day and inter-day assays of hinokitiol are shown in Table 2. In the intra-day assay, the range of standard deviation was within 5.2% to 7.7% of the mean, and recoveries were within the range of 93.3%
![]()
Table 1. Sensitivity of various methods for determination of hinokitiol.
![]()
Table 2. Intra- and inter-day assay reproducibility for determination of hinokitiol.
to 106.3%. In the inter-day assay, the range of standard deviation was within 6.3% to 8.8% of the mean, and recoveries were within the range of 90.4% to 104.0%.
3.5. Interference
Cosmetics generally contain several parabensas preservatives [12] [13] [14] . Therefore, interference with the detection of Dabsyl-hinokitiol derivative by seven parabens (methyl 4-hydroxybenzoate, ethyl 4-hydroxybenzoate, propyl 4-hydroxybenzoate, isopropyl 4-hydroxybenzoate, butyl 4-hydroxybenzoate, isobutyl 4-hydroxybenzoateand benzyl 4-hydroxybenzoate, each 0.2 μg/mL in 1% acetonitrile) was investigated. Derivatization was performed as described above, and the running time was set at 35 min. Table 3 summarizes the retention times of the seven Dabsyl-paraben derivatives (Dabsyl-methyl 4-hydroxy- benzoate, Dabsyl-ethyl 4-hydroxybenzoate, Dabsyl-isopropyl 4-hydroxybenzoate,
![]()
Table 3. Retention times of paraben derivatives tested for interference with Dabsyl-hi- nokitiol peak.
Dabsyl-propyl 4-hydroxybenzoate, Dabsyl-benzyl 4-hydroxybenzoate, Dabsyl- isobutyl 4-hydroxybenzoate and Dabsyl-butyl 4-hydroxybenzoate). A slight peak (under lower limit of quantification of hinokitiol) was observed at 6.8 min, and the retention times of the major peaks of the seven Dabsyl-paraben derivatives were more than 11.5 min. These data suggests that parabens would not interfere with hinokitiol determination in this system. Thus, Dabsyl-Cl is a more appropriate derivatization reagent than NBD-F.
3.6. Analysis of Skin Lotions
Figure 6 shows typical chromatograms obtained from skin lotions A and B. The peak of Dabsyl-hinokitiol was detected at 6.8 min in both samples A and B.
The established method was used to determine hinokitiol concentration in skin lotion sample A and in samples spiked with standards. As shown in Table 4, the concentration of hinokitiol in the skin lotion was found to be 559 ± 56 μg/mL (range, 499 to 614 μg/mL). Skin lotion A contained hinokitiol at 0.05 g/100 mL according to the label. It is possible that parabens contributed to the slight difference, because peaks of paraben derivatives were observed at 16.1 and 26.3 min (data not shown). Recovery of spiked hinokitiol from the cosmetic was 87.4% ± 5.7% (range, 83.2% to 96.1%). Thus, our result is consistent with the labeled concentration. Next, the established method was used to determine hinokitiol in skin lotion sample B and in samples spiked with standards. No peaks due to parabens were observed (data not shown). The concentration of hinokitiol in the skin lotion was found to be 76.2 ± 2.5 μg/mL (range, 73.1 to 78.3 μg/mL). Recovery of spiked hinokitiol was 86.2% ± 3.5% (range, 83.7% to 91.3%).
4. Conclusion
We have developed a simple HPLC-VIS method for determination of hinokitiolin skin lotion after pre-column derivatization with Dabsyl-Cl. The sensitivity
![]()
Figure 6. Typical chromatograms of skin lotion sample ((a), 0.25 mL/20mL) and skin lotion sample ((b), 1.0 mL/20mL) after derivatization with Dabsyl-Cl. Samples were reacted with Dabsyl-Cl for 10 min in borate buffer, pH 9.5, at 55˚C. Retention time of Dabsyl-hinokitiol derivative: 6.8 min (arrowed peak).
![]()
Table 4. Level of hinokitiol in skin lotions (A and B) and recovery of spiked hinokitiol (100 μg).
of the assay can be classed as moderate. This system showed little interference from parabens, in contrast to the previously reported assay using NBD-F [9] . The new system should be suitable for quality-control monitoring of hinokitiol levels in cosmetics containing parabens.