1. Introduction
Generally, tungstate is a kind of important inorganic functional material. AWO4 (A = Ba, Sr, Ca, Pb) tetragonal scheelite-type crystals of divalent metal ion tungstate have been of immense interest because of their remarkable properties such as luminescence, nonlinear optical activity, photocatalysis, and scintillation [1] - [16] . Many scholars have been interested in the study of the luminescence properties of it. The tungstate is a typical self-activated luminescent material. Its light emission originates from the 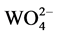 complex ion, and it can emit highly efficient fluorescence under the excitation of X-rays, ultraviolet light and the cathode-ray. The luminescence spectrum of the tungstate is very stable, and the spectral band of the intrinsic luminescence spectrum is very wide, which accounts for most of the visible light region. Because the tungstate crystal has the advantages of high luminescence efficiency, high density, strong anti-radiation ability, and so on, it attracts people’s attention. There are many methods to prepare the tungstate nanomaterial, such as hydrothermal method, sol gel method, high temperature solid state method, template method, etc. Microwave synthesis is a simple method. It has the characteristics of fast and high energy efficiency. Therefore, we choose microwave synthesis method to target samples.
complex ion, and it can emit highly efficient fluorescence under the excitation of X-rays, ultraviolet light and the cathode-ray. The luminescence spectrum of the tungstate is very stable, and the spectral band of the intrinsic luminescence spectrum is very wide, which accounts for most of the visible light region. Because the tungstate crystal has the advantages of high luminescence efficiency, high density, strong anti-radiation ability, and so on, it attracts people’s attention. There are many methods to prepare the tungstate nanomaterial, such as hydrothermal method, sol gel method, high temperature solid state method, template method, etc. Microwave synthesis is a simple method. It has the characteristics of fast and high energy efficiency. Therefore, we choose microwave synthesis method to target samples.
In this study, we report on a direct feeding microwave synthesis method to synthesize CaWO4 of homogeneous double cone structure. As-prepared samples have strong luminescence properties, and it has purity green emission at 495 nm and 521 nm.
2. The Experiment
2.1. Synthesis of BaWO4 of Double Cone Structure
All chemicals were analytical grade and used without further purification. Nano- double cone of BaWO4 were prepared by a direct feeding microwave synthesis method. In a typical procedure, 2.61 g of BaNO3 was dissolved in 50 ml of 2% PEG aqueous solution, dispersed and dissolved with ultrasonic waves, mixed uniform for A solution. 3.30 g of Na2WO4∙2H2O was dissolved in 50 mL of 2% PEG aqueous solution, the dispersion was dissolved by ultrasonic mixing, for B solution. The A, B solutions were mixed rapidly transferred into 250 ml of flask, then the mixed solution was placed in a microwave refluxing system to react for 20 min with a power microwave radiation of 40% and cool down naturally to the room temperature [17] . Then the precipitate was centrifuged, washed with the deionized water for several times and dried at 60˚C in the vacuum for 8 h. The final product was collected for the characterization.
2.2. Characterization [17]
The crystal structure of Nano-double cone of BaWO4 was measured by XRD on a Shimadzu XRD-6100 X-ray diffractometer (Cu Ka radiation, l = 0.15418 nm). The morphology and size of products were determined by SEM. The SEM images were recorded on a Quanta 200 FEG field emission scanning electron microscope. The optical property was obtained by Cary Eclipse fluorescence spectrometer (USA Varian Company).
3. Results and Discussion
The XRD pattern of the as-prepared sample is shown in Figure 1. All the peaks (peak 2θ: 26.71, 32.15, 43.22, 45.99, 53.87, 54.75) including the minor ones are indexed for a perfect tetragonal scheelite (JCPDS File No. 43-0646). The diffract-
![]()
Figure 1. X-ray diffraction pattern of as-prepared samples with PEG.
tion peak is strong and sharp, which indicates that the sample has a high degree of crystallinity.
Figure 2 shows the SEM image of as-prepared sample. It shows that the majority of the as-prepared sample is a homogeneous double cone structure, and some particles attached to it. The length of most of double cone is 10 µm.
Figure 3 is photoluminescence spectrum of as-prepared sample. The excitation wavelength is 235 nm. It can be seen in the 363, 425, 434, 495 and 521 nm have a certain luminescence, which is the strongest at 495 nm, followed at 521 nm. At 450 - 490 nm has a very high peak, this is a frequency doubling peak of excitation light.
We also investigated the effect of different surfactants on the luminescence properties. Figure 4 is photoluminescence spectra of samples with different surfactants. It can be seen that at 495 nm, the nonionic surfactant PEG is the best, next is the anionic surfactant sodium dodecyl sulfate (SDS) times, the cationic surfactant cetyltrimethyl ammonium bromide(CTAB) is worst. But at 521 nm, next is CTAB, SDS is worst.
With different surfactants, the morphology of the as-prepared samples are different, the results are shown in Figure 5. Figure 5(a) shows SEM image of sample under the synthesis condition of presence of PEG; Figure 5(b) shows SEM image of sample under the synthesis condition of presence of CTAB; Figure 5(c) shows SEM image of sample under the synthesis condition of presence of SDS.
4. Conclusion
BaWO4 of a homogeneous double cone structure was successfully prepared by a
![]()
Figure 2. Scanning electron micrograph image of as-prepared sample with PEG.
![]()
Figure 3. Photoluminescence spectrum of as-prepared sample with PEG.
![]()
Figure 4. Photoluminescence spectra of as-prepared samples with different surfactant.
![]()
![]()
![]()
Figure 5. Scanning electron micrograph image of as-prepared sample with different surfactant.
direct feeding microwave synthesis method. This method is a simple, fast, energy-efficient way.
As-prepared samples have strong luminescence properties, and it has purity green emission at 495 nm and 521 nm. The luminescent intensity of samples synthesized by different surfactants is different. When PEG2000 is as surfactant, the luminescence intensity of as-prepared sample was maximum.