Aesthetic Outcome after Nipple Sparing Mastectomy for Cancer Patient Egyptian Pattern ()
1. Introduction
Breast cancer is a devastating disease affecting women of all ages worldwide with the age incidence in Egypt being one decade younger than the mean age incidence [1] . This has a significant impact on our young patients and dramatically affects their quality of life. Recent advances in breast reconstruction and the introduction of various Oncoplastic techniques have resulted in significant improvements in the quality of life along with the psychological well-being of most of our patients. In Egypt, 41 percent of women with symptomatic breast cancer and 75 percent of those with screening detected breast cancers are treated with conservative breast surgery [2] [3] [4] . Despite this, many women may require mastectomy for multicenteric disease and extensive widespread DCIS or suspicious microcalcifications. Additionally, advances in clinical genetics and increased awareness of breast cancer risk factors have resulted in an increased group of women requesting prophylactic mastectomy for risk reduction. In an attempt to decrease morbidity and improve functional outcome, 1960 witnessed the surgical evolution from the radical mastectomy to the modified radical mastectomy and in 1969 the WHO approved randomized control trial comparing radical mastectomy to quadrentectomy [5] . Then, it came the idea of skin sparing mastectomy described by Toth and Lambert in 1991 [6] . The natural progression from skin preservation would be nipple-preservation.
The nipple is the focal point of the breast and its reconstruction is often cited by women as making their breast reconstructions complete [7] . However, there are problems with reconstructed nipples, the greatest being loss of projection over time and the need for tattoos to provide pigmentation of both nipple and areola that fade over time. Reconstructed nipples are insensate and not erectile, and patient satisfaction is variable [8] . Nipple-sparing mastectomy includes removal of the tissue located behind the nipple-areola complex (terminal ductal system) [pic1] to decrease the risk of recurrence in the nipple-areola complex, reported rates of which range from 12 to 48 percent [9] , which puts the nipple at risk for ischemic necrosis because it is stripped of local perforators, leaving it dependent on the dermal microvasculature. However, the preservation of the NAC along with the native skin envelope and infra-mammary fold allows the immediate reconstruction of the breast to give optimum cosmetic outcome. The purpose of this study is to evaluate the influence of incision choice and the type of reconstruction on aesthetic outcome, complications and nipple necrosis rates.
2. Patients and Methods
74 patients with breast cancer underwent NSM using different approaches with immediate reconstruction using extended LD flap with or without implant augmentation from the period of January 2013 to November 2015. Axillary staging using SLN was done for 18 patients and formal axillary lymph node dissection for 51 patients and there were no need for axillary management in 5 patients. Patients included in the study were female patients aged 20 to 60 years old, without any serious medical co-morbidities, desiring immediate breast reconstruction post mastectomy for multicenteric breast cancer (T1 to T3), Patients with wide spread suspicious micro-calcifications. Provided that tumors lie at a distance of 2 cm or more from the central quadrant of the breast with negative intra-operative tumor involvement of terminal ductal system via pathological frozen section assessment. Patients excluded from the study were those refusing immediate breast reconstruction, Patients with severe medical co-morbidities and /or special habits (Cardiac, uncontrolled DM and heavy smokers), patients having central tumors, inflammatory breast cancer or peaud’orange and if the terminal ductal system proved to be positive via intra-operative pathological frozen assessment.NSM was done through either Elliptical, Lateral, Peri-areolar or Infra-mammary incision. Choice of incision was based on Tumor location, previous biopsy site or scar and the shape and size of the breast. The data recorded included: Operative details, operative time, feasibility of resection of all glandular tissue and the need for separate axillary incision. Post-operative complications: skin envelop necrosis, flap necrosis, NAC necrosis, donor site hemorrhage, seroma formation, donor site wound gapping and wound infection. Aesthetic outcome was evaluated depending on 5 aesthetic criteria including breast size, shape and volume, scar visibility and nipple position. Evaluation was done through a subjective method of assessment: patient self-evaluation along with three independent surgeons evaluations. Time of evaluation: immediately after surgery, delayed evaluation within 2 to 3 months after surgery and evaluation after end of adjuvant therapy. Medical photos were taken at each stage for documentation.
2.1. Ethical Issues
Consent forms signed by all patients before enrollment into the study along with the approval of the ethical committee. Subject identification and protection of confidentiality were assured as; Access to medical files was restricted to the individuals list in this study, no reference of patients’ possible identifiers were included in the results, also no facial photography along with obtaining patients consents on the medical photos.
2.2. Statistical Methods
Data management and statistical analysis was performed using statistical package for social sciences (SPSS) vs.21. Numerical data was summarized using means and standard deviations or medians and ranges as appropriate. Categorical data was summarized as percentages. Kappa statistics as a measure of agreement beyond chance between observers rating of aesthetic outcomes was calculated. Wilcox signed rank test was used to compare improvement and deterioration after end of adjuvant treatment as to that immediately after surgery. P value always two tailed and is significant at 0.05 levels.
3. Results
The median age of the patients was 29 years (range: 24 - 46 years) with mean age (30.64) and standard deviation (5.44). 39 cases (52.7%) were in the right side and 35 cases (47.3%) were in the left side. Pathological results: 5 patients had phylloides tumor, 5 patients had ILC, 9 patients had IDC with diffuse DCIS and 55 patients had multicenteric IDC with variable percentage of intra duct component. Aside from the phylloides cases 69 cases were classified as luminal A. Staging: 7 patients were staged as T1N0, 10 patients were T2N0, 33 patients were T2N1 and 19 patients were T3N1 (Table 1). The tumor proximity from the nipple areola complex ranged from 2 to 4 cm clinically and confirmed by mammography. Intra-operative frozen section examination of the terminal ductal system in the retro-areolar tissue was performed to decide whether the NAC was positive for tumor cells (Picture 1). No involvement of the nipple core or areola was found on frozen section biopsy in any patient. The incisions used were elliptical (37.8%), lateral (27%), peri-areolar (21.6%) and inframammary (13.5%) incisions (Pictures 2-5). Immediate breast reconstruction using autologous tissue
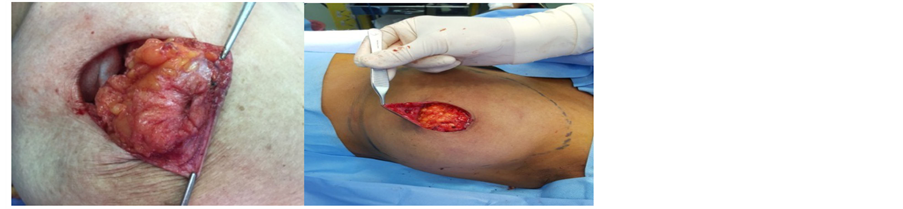
Picture 1. Excision of terminal ductal system from the back of the nipple.
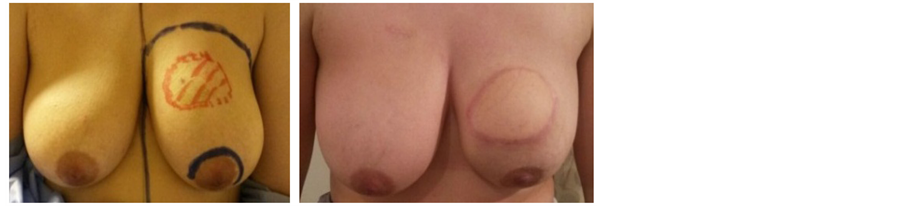
Picture 2. Pre- and post-operative pictures of nipple sparing mastectomy through an Elliptical incision.

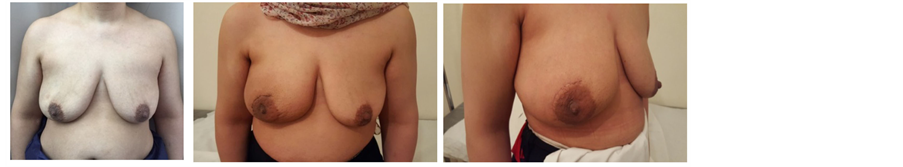
Picture 3. Pre- and post-operative pictures of NSM through a lateral incision.
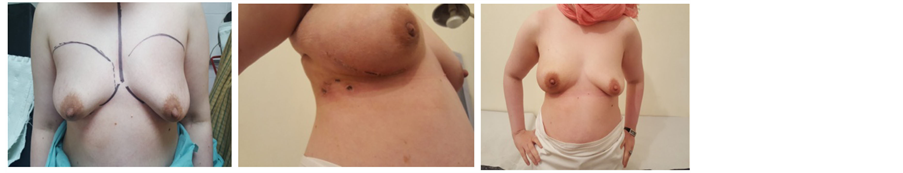
Picture 4. Pre- and post-operative pictures of nipple sparing mastectomy through a peri-areolar incision.
![]()
Table 1. Pathological and surgical characteristics of the study.
Picture 5. Pre- and post-operative pictures of nipple sparing mastectomy through an infra-mammary incision.
was performed in 81.1% of patients using only extended LD flaps and in 18.9% of patients the flaps were augmented using silicone implant insertion beneath the flap. Formal axillary lymph node dissection was done in 68.9% of patients and SLN was done in 24.3% of patients. 25.7% of patients received neo-adjuvant CTH, 83.8% of patients received adjuvant CTH and 70.3% of patients received adjuvant RTH. The median resection time was 55 minutes (range: 40:75 minutes) and median flap harvesting time was 55 minutes (range: 45:80 minutes) and median flap insertion time was 70 minutes (range: 45:100 minutes)and the axillary median evacuation time was 40 minutes (range: 15:50 minutes).The recorded postoperative complications were: nipple areola complex sloughing in 13.5% of cases (Picture 6), donor site hemorrhage in 5.4% of cases, donor site seroma formation 33.8%, donor site wound infection in 5.4% of cases, breast wound infection in 6.8% of cases and exposure of implant in 2.7% of cases (Table 1). The median follow-up was 28.5 months (range: 18−38 months) during which no local recurrence or systemic disease were recorded. Assessment of aesthetic results was based on periodic assessment: Immediately after surgery, 3months after surgery and after the end of adjuvant treatment. Using a subjective method of assessment, a scoring system from 1to 5assigned by the patient, the surgeon, an independent oncoplastic surgeon and a plastic surgeon which was stratified by subscales according to Lowery et al. [10] . Breast shape, volume, ptosis and symmetry were defined as excellent, very good, good, fair, and poor according to the total score (Tables 2-5). Regarding agreement on assessment for aesthetic results between second surgeon and plastic surgeon, for immediate evaluation there was an agreement in breast size with kappa 0.74 and p value < 0.001, breast shape with kappa 0.48 and p value < 0.001, scar visibility with kappa 0.67 and p value < 0.001, nipple position with kappa 0.89 and p value < 0.001, skin color with kappa 1 and p value< 0.001, donor site with kappa 1 and p value< 0.001.Agreement in evaluation 3 months after surgery was in breast size with kappa 0.78 and p value < 0.001, breast shape evaluation with kappa 1 and p value < 0.001, scar visibility evaluation with kappa 0.97 and p value < 0.001, nipple position evaluation with kappa 0.95 and p value < 0.001, skin color evaluation with kappa 0.85 and p value < 0.001, donor site evaluation with kappa 1 and p value< 0.001. Agreement in evaluation after finishing adjuvant treatment was in breast size with kappa 1 and p value < 0.001, breast shape evaluation with kappa 1 and p value < 0.001, scar visibility evaluation with kappa 1 and p value < 0.001, nipple position evaluation with kappa 1 and p value < 0.001, skin color evaluation
![]()
Table 2. Change in patient self-assessment for aesthetic results 3 months after surgery and after adjuvant treatment regarding improvement and deterioration.
*P value is significant ≤ 0.05. #statistical significance is for comparing patient evaluation after adjuvant treatment as different from immediately after surgery.
![]()
Table 3. Change in first surgeon assessment for aesthetic results 3 months after surgery and after adjuvant treatment regarding improvement and deterioration.
*P value is significant ≤0.05. #statistical significance is for comparing surgeon evaluation after adjuvant treatment as different from immediately after surgery.
![]()
Table 4. Change in onco-plastic surgeon assessment for aesthetic results 3 months after surgery and after adjuvant treatment regarding improvement and deterioration.
*P value is significant ≤0.05. #statistical significance is for comparing onco-plastic evaluation after adjuvant treatment as different from immediately after surgery.
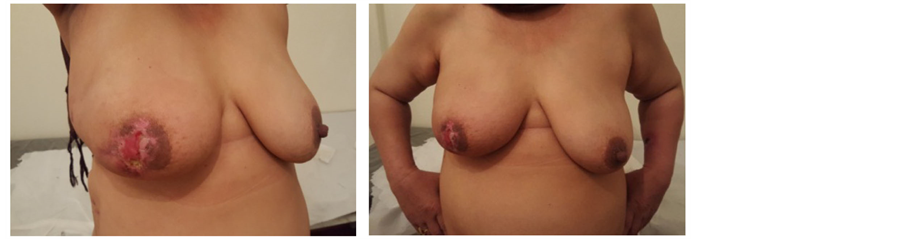
Picture 6. Superficial sloughing of the nipple areola complex.
![]()
Table 5. Change in plastic surgeon assessment for aesthetic results 3 months after surgery and after adjuvant treatment regarding improvement and deterioration.
*P value is significant ≤0.05. #statistical significance is for comparing plastic surgeon evaluation after adjuvant treatment as different from immediately after surgery.
with kappa 1 and p value< 0.001, donor site evaluation with kappa 1 and p value < 0.001.
4. Discussion
Our series of 74 NSMs were performed at the Egyptian national cancer institute through different approaches: 28 through elliptical incision (Picture 3), 20 through incision in lateral mammary fold (Picture 4), 16 through periareolar incision (Picture 5) and 10 through inframammary incision (Picture 6). The choice of incision remains a point of debate, although it is more attractive to choose the hidden and less visible incisions that offer the best post aesthetic outcome. Every incision has its advantages and limitations. Our high rate of elliptical incisions is explained by the initial presentation of patients with previous contaminated miss placed scars, biopsy sites and hematomas requiring removal of an area of the native skin envelope (Picture 2). This incision although much easier in resection, carried the worse cosmetic outcome in terms of scar visibility due to the high rate of hypertrophic scar and keloid formation in Egyptian patients. The inframammary incision had a very good post-operative cosmetic outcome and a low incidence of NAC necrosis, however, access to the back of the nipple was achieved after elevation of the lower skin flap. In the incidence of having a positive result for the terminal ductal system would result in a mastectomy rather than a conversion to a skin sparing mastectomy. The lateral incision gave very good to excellent cosmetic out come with a relatively early reach to the back of the nipple and excellent exposure to the axilla, it is also extendable to the infra mammary fold however it was more technically difficult in patients with larger and more ptotic breasts to reach the medial and infro-medial limits of resection in addition to being the most difficult type of incision for flap placement during the reconstructive phase. The periareolar incision on the other hand offered the earliest access to the back of the nipple and the easiest to convert to a relatively acceptable type I skin sparing mastectomy on a positive intra-operative frozen section result. It also offers a hidden incision, thus very good to excellent postoperative cosmetic result. The proximity of the incision line to the nipple carries a higher risk of NAC sloughing, and being a small incision, comes with the disadvantages of a key hole incision, but with lighted retractors and good exposure, we did not find the need to neither extend this incision for the resection access to the axilla nor the insetting of the flap. Immediate reconstruction was done with extended LD flaps alone in 60 cases and with implants in 14 cases. We reported postoperative superficial sloughing and nipple necrosis in 10 cases (13.5%), which was mainly related to prolonged retraction on the breast skin envelop during the resection and axillary evacuation. Unfortunately, there was an increase in incidences of seroma formation in both the donor site 33.8% and resection site 13.5% which decreased in donor site after quilting of the lower flap. All the patients in our study were followed up for at least 18 months after surgery, and the median follow-up period was 28.5 months (range: 18 - 38 months).There was no local recurrence or systemic disease. The two major issues to be addressed are: the oncological safety and the technical approach. Data continue to show equivalence of these conservative techniques to the more traditional modified radical mastectomy in terms of local and regional recurrence rates [11] [12] . The issue of a greater risk of local recurrence (LR) has been addressed by different authors [13] . Tumor involvement of the NAC has been overestimated in the past and this has led some surgeons to attempt preservation of the NAC in view of obtaining better cosmetic results [14] [15] . In a retrospective series of 286 NSM, 16 (5.6%) were found to contain tumor in the NAC [14] . Another series of 217 mastectomy specimens by Simmons et al. reported NAC tumor involvement in 23 cases (10.6%) [15] . In contrast to these results, one report found that the NAC was involved in 58% of mastectomy specimens [16] . The incidence of nipple involvement by 10.6% of the patients after NSM described by Simmons et al., can reach 58% in tumors bigger than 4 cm or located closer than 2 cm from the nipple [14] [15] [16] . Such high incidences is consequently a hindrance for considering nipple-sparing mastectomy as a reasonable option for all breast cancer patients. Various incisions have been used for NASM by different authors in their reported series [17] [18] [19] [20] . Garwood et al. in their study of total skin sparing mastectomy found that the use of inframammary incisions is an excellent approach for small- or medium-sized breasts and, if enlarged, works well for large breasts as well [21] in our series it is excellent for small sized breasts, relatively good in medium sized breast, but poor in large breasts and this was due to larger degree of ptosis among Egyptian women with large breasts. We select patients with breast lesions away from areolar margin by at least 2cm with negative intra-operative frozen assessment of terminal ductal system after conning of the nipple. The low rate of local recurrence is supported by the results of previous studies, which have confirmed the oncological safety of NSM. For example, Omranipour et al. (2008) concluded that NSM is oncologically safe for early breast cancer (stages 0-II) [24] . Sookhan et al. concluded no local recurrence at a median follow-up of 10.8 months after NSM for 18 cases [25] . Caruso et al. reported only 2% local recurrence rate within the NAC in a series of fifty NSMs for breast cancer [26] . Rusby et al. have published the most recent review of NSM in the literature [27] . They also found recurrence rates of less than 5% in properly selected patients undergoing NSM for breast cancer treatment. In our series among all cases with median follow-up period was 28.5 months (range: 18 - 38 months).There was no local recurrence or systemic disease.
Assessment of aesthetic results was based on periodic assessment; immediately after surgery, 3months after surgery and after finishing adjuvant treatment, via medical photography assessed by the patient, via subjective aesthetic assessment; the surgeon, an oncoplastic surgeon and a plastic surgeon and via an objective aesthetic assessment, which was stratified by subscales according to Lowery et al. [10] . Breast size, breast shape and volume, scar visibility, nipple position, skin color and donor site results were defined as excellent, very good, good, fair, and poor according to the total score. Various subjective and objective scores have been used evaluating aesthetic outcomes after immediate breast reconstruction following NSM. Salhab et al. assessed the patient’s satisfaction with the outcome of surgery with a detailed questionnaire including a linear visual analogue scale ranging from 0 (not satisfied) to 10 (most satisfied) [28] . Salgarello et al. evaluated the reconstructive and aesthetic outcomes by clinical examinations and by reviewing the clinical pictures of the breasts [29] . More than 90% of patients in our study had good or excellent Lowery scores at 10 months of follow-up. More- over, the patient satisfaction as assessed by questioning the patients about their satisfaction with the aesthetic results of surgery was acceptable in all the cases. Regarding agreement on assessment for aesthetic results many authors satisfied with patient self-assessment and surgeon assessment and conclude the agreement on assessment between them [3] [9] [10] [17] [18] [20] [22] [23] [25] [29] , in our series we thought that to avoid biased results the agreement on assessment for aesthetic results was done by non-involved professional surgeons in this study who were the plastic surgeon and an onco-plastic surgeon.
5. Conclusion
NSM is a safe and technically feasible procedure that offers adequate oncologic results along with very good cosmetic outcome. Choice of incision and method of reconstruction should be individually tailored to suit each patient. Breast cancer patients can benefit from sound resection and enjoy a sense of wholeness.
Funding
This research didn’t receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflicts of Interest
The authors have no potential conflict of interest to disclose.
Abbreviations
BCS: breast conservative surgery.
NSM: nipple sparing mastectomy.
LD: latissmus dorsi.
NAC: nipple areolar complex.
IDC: invasive ductal carcinoma.
ILC: invasive lobular carcinoma.
DCIS: ductal carcinoma in situ.
CTH: chemotherapy.
RTH: radiotherapy.