New Petrological and Geochemical Data of the Nephelinitic Lavas and Geodynamic Implications of Mount Etinde (Cameroon) ()
1. Introduction
Located near Batoke village (Figure 1), Mount Etinde is unique among the many volcanoes along the 1600 km long Cameroon Hot Line. They erupted at the very limit between the ocean and the continent, where fracturation (fractures at the continent-ocean boundary and regional N308E and N708E zones) is more intense and deeper [1] . Hyperalkaline rocks such as nephelinitic are rare, being less than 1% of all igneous rocks on the Earth [2] . Mount Etinde consists mainly of nephelinitic lavas. The lavas are characterized by a complex mineralogy and by the occurrence of rare carbonate [3] . Some nephelinites contain similar calcite in size and shape to those describe in Oldoinyo Lengai [4] . The main mafic minerals are clinopyroxene (diopside), olivine and wollastonite. Nepheline bearing rocks, present, occur in significant volume and might be of recrystallization origin. Other minerals present include characteristic magnesian spinel and perovskite. The common accessories are sphene and Fe-Ti oxides. The main products of hydrothermal alteration and/or weathering are zeolites and analcime and cancrinite. In this paper, we present new petrological, mineralogical and geochemical data (major and trace elements) on the main rock types of Mount Etinde, in order to describe the magmatic processes that led to their formation.
1.1. Geological Framework
The Cameroon Hot Line is interpreted as a megashear zone, developed on both oceanic and continental domains [1] . The structure is characterized by a SW-NE alignment (trending 30˚) of volcanic massifs and plutons extending from Pagalu Island to Lake Chad for more than 1600 km. The general geology of Mount Etinde has been extensively described by [5] - [12] and the more recently summarized and updated by [13] [14] [15] . Its basement (Pan-African granite and gneisses) is covered by cretaceous to quaternary sediments [16] . The nephelinitic lavas erupted less 1Ma ago, and is contemporary with recent lavas of Mount Cameroon [17] . These rocks contain nepheline, clinopyroxene, garnet, melilite and post magmatic minerals such as sodalite, zeolite and analcite. Olivine occurs mostly in the olivine nephelinite where it makes up less 5 wt%. Varieties of nephelinites are named on the basis of the dominant mineral and nepheline occurs.
1.2. Analytical Method
Mineral compositions were studied in detail and the compositional ranges given below are based on electron microprobe (CAMEBAX SX 50 at the University of Marie Curie, Paris) measurement of mafic phases in only a few representative sample of nephelinite. The measurement were made according to standard analyzed data, under the condition expressed in kv (acceleration) nA (beam current) and (counting times at the peak). Olivine (15 kv, 40 nA, 20s and Si, clinopyroxene (15 kv, 40 nA, 20 s for Si, Al, Fe, Mg, Ca, Na Mn and 30 s for Ti and Zr), nepheline (15 kv, 40 nA, 15 s for Si, Al, and 20 s for Ca, Na, K ), garnet (15 kv, 40 nA, 20 s for Si, Al, Fe, Mg, Ca, Na, Mn and 15 s for Ti), melilite ((15 kv, 40 nA, 20 s for al elements), perovskite and sphene (15 kv, 40 nA, 5 s for all
![]() (a)
(a)![]() (b)
(b)
Figure 1.(a) Location of the study area, tectono-magmatic sitting of Mount Cameroon [1] , (b) modified map of Etinde massif [12] .
elements), (oxides (15 kv, 40 nA, 20 s for Ti, Fe, Mn, Mg, 20 s for Si, 20 s for Cr and 30 s for Al) natural silicates and oxides as standards, PAP corrections were made using CAMECA software [18] . Whole rock chemical analyses of peralkaline nephelinite from Etinde massif were carried out at the “Centre de Recherches Pétrographiques et Géochimiques” in Nancy. Major elements were analyzed by ICP-AES and trace elements by inductively coupled plasma mass spectrometry ICP-MS by Ngounouno.
1.3. Experimental Methods
Thermodynamic condition of crystallization during the eruption of Etinde nephelinite was determined by the QUILF95 software [19] . The programs application (QUILF thermobarometer) is explained by many works [20] [21] [22] . The minerals used for calculations, had not attained subsolidus re-equilibrium as showed by their unzoned and homogeneous compositions. Oxygen fugacity (fO2) was calculated using the equation ∆logFMQ = logfO2 − FMQ (∆logFMQ ≤ 0). The experimental aSiO2 was calculated using the QUILF95 software from coexisting clinopyroxene, olivine and titano magnetite (aSiO2 = 0.30 - 0.60). The experiments with an acceptable error (±0.02) gave the lowest values for aSiO2. Geothermobarometric calculations based on the composition of olivine, clinopyroxene and Fe-Ti oxide was used to determine the temperature range from 950˚C to 1250˚C at lower fO2.
1.4. Petrography
Our description of the nephelinite is based on samples collected from the flank of Mount Etinde and the Batoke beach, possibly representing the volcanic event. The textures are generally porphyritic (15% - 30% phenocrysts), with groundmass exhibiting various textural types including intergranular, intersertal. Photomicrograph, taken under crossed polarizers, of nephelinte rocks shows olivine, clinopyroxene, nepheline and garnet phenocrysts. The photograph, taken plane polarized light, shows the typical shape of olivine and cliopyroxene: the irregular cracks and slight alteration along the craks (Figure 2(a) and Figure 2(c)). Clinopyroxene and nepheline are birefringent, garnet is black. The most nephelinitic lavas contains phenocrysts of Ca-rich clinopyroxene (<20 vol%), titanomagnetite (<2 vol%) and in some samples, melilite (<5 vol%). Nepheline phenocrysts occurring in some of these nephelinitic rocks is automorphic (Figure 2(b)). They are coarse grained and consist of euhedral to subhedral nepheline (typically replaced by cancrinite alteration product), altered formed haüyne or nosean, and alkali feldspar. Some olivine crystals are surrounded by rims of diopside. These rocks contain rare, resorbed phenocrysts of olivine surrounded by clinopyroxene as well as magnetite. The clinopyroxene phenocrysts are subhedral to euhedral and reach a few millimeters in size. At the contact with these interstitial domains, the brown diopside phenocrysts are transformed into green hedenbergite with a composition that matches that of groundmass pyroxene. Some clinopyroxene show zoning (Figure 2(a)). This characteristic has previously been reported, [9] [12] [13] [23] . Ti-magnetite is common, and partly altered olivine and haüyne are present in haüyne nephelinitic and
![]() (a) (b)
(a) (b) ![]() (c) (d)
(c) (d)
Figure 2. Photomicrograph (plane polarized light) of layer, illustrating the occurrence of oxyde. Garnet, (grt), cancrinite (cn), nepheline (ne) and clinopyroxene (cpx) are also in the field of view.
haüynophyre. Spinel, described here from the nephelinitic lavas, occurs as tin (up to 2 mm) opaque or brownish euhedral inclusions in olivine and euhedral inclusions in aluminous clinopyroxene. Spinels found are xenomorphic, probably because it crystallized late. Small magnetites are irregularly distributed between the silicates, the locally form lenticular zones of interstitial Ti-magnetite. The groundmass consists of prismatic clinopyroxene and nepheline up to 0・2 mm in length microphenocrysts set in a matrix with smaller crystals, perovskite, magnetite, sphene and carbonate. The prismatic Zeolite is automorphe in LPA and replaced by analcime product (Figure 2(d)). The minor and accessory phases include zeolite, analcime, magnetite, apatite and perovskite. In additional, spinel is the most common accessory mineral but it is usually pale to dark brown. Groundmass is frequently altered and consists of a low temperature assemblage of zeolite, which probably replace feldspar ± nepheline. Melilite microphenocrysts occur in a green-grey aphanitic textural in melilite nephelinite. Groundmass clinopyroxene grains are up to 0.5 mm in size.
2. Mineral Chemistry
Selected electron microprobe analyses of pyroxene and garnet phenocrysts are represented in the figure. These data are plotted on conventional Diopside-Hedenbergite-Enstatite- Ferrosilite and Almandine-Pyrope-Spertartine quadrilateral and ternary diagrams, respectively, comparing the current results against previously published data from the Etinde Mountain [11] [12] [13] .
Rage homogeneous olivine grains in the néphélinite are Mg-rich (Fo86) and moderately high levels of CaO content solid-solution, and are similar composition to olivine grain from xenolith
2.1. Pyroxene
The nomenclature of [24] was used for pyroxene. Chemical analyses of pyroxene were plotted on the quadrilateral classification diagram Di-Hd-En-Fs. The result reveals three clinopyroxene types in the Etinde massif, magnesium-rich diopside Wo49-52En23-47Fs4-17, hedenbergite Wo50-47En13-11Fs37-32 and augite Wo44-47En17-23Fs14-17 (Table 1), the diopside has Mg# [=atomic 100 × Mg2+/(Mg2+ + Fe 2+ + Mn2+)] ranging between 0.63 and 0.71). Moreover, clinopyroxene crystals display a trend of decreasing En and Wo with increasing Fs on the quadrilateral classification diagram. Clinopyroxene is low in calcium, 0.73 - 0.83 atoms per formula unit (a.p.f.u.), and contains significant Al (0・23 - 0・39 a.p.f.u.). It consists of a relatively large diopsidic core that is relatively Ca-poor and Na-rich, and exhibits large differences in Al content from grain to grain (mg-number 0.78 - 0.83) (Figure 3).
![]()
Figure 3. The quadrilateral classification diagram of clinopyroxene [24] .
![]()
Table 1. Clinopyroxene in pyroxene nephelinite.
2.2. Garnet
Garnet composition falls within the compositional range of garnets reported in other lavas and ijolites from Oldoinyo Lengai [25] , and nephelinite from Tanzania [3] . There are no significant differences in the compositions of the garnets from the different nephelinite varieties at Etinde. The main compositional variations are in the amounts of Ti and total Fe, which vary inversely. The minor elements, MnO (<1%) and ZrO2 (<1%), present concentrations in appreciable amounts. Garnet is andratite rich, with an overall compositional range of And72-43Pr7-11, Uva0,1-0.54Sps4-10, and Grs13-44 (Figure 4). The TiO2 contents are low, varying from 7 to 14 wt%.
2.3. Haüyne
Representative chemical analyses and structural formulae (O2− = 21) of haüyne is presented in Table 2. Ternary diagram (Figure 5) shows that haüyne data are similar to those of pan de AZUCAR haüyne [27] . The diagram of the system reveals that Haüyne has CaO concentration ranging between 60 and 80 wt%.
2.4. Perovskite and Sphene
Perosvkite and Sphene are trace- and rare element-rich. The Trace elements content
![]()
Figure 4. Ternary classification diagrams after [26] .
![]()
Figure 5. Ternary classification diagrams of feldspathoid after [28] . The round and star represent respectively haüyne of Mount Etinde and the lavas flow in Pan de Azúcar 2 series [27] .
![]()
Table 2. Major and minor element compositions of Haüyne phenocrysts of Haüynopyre.
(BaO, SrO, ZrO2 and Nb2O3) represented slightest 4.0 wt%. Sphene’s rare elements content is low ≈ 2 wt%, and for perovskite they vary between 3 - 8 wt%. The ternary classification CaO-SiO2-TiO2 diagrams shows that the crystallization temperature condition of sphene is low compared to perovskite [29] . The phase diagram of the system reveals that perosvkite contains 40 wt% Cao and 60 wt% TiO2.
3. Nepheline
Phenocryst cores contain around 19 - 22 mol % kalsilite (Ks) and generally contain excess Si. Although some cores are homogeneous, others are complex, with zones containing differing K and Fe concentrations. Enrichment in K and Fe is a feature of the rims seems of nepheline described by [25] . Over all, the nepheline in olivine nephelinite is more potassic (around 25% - 27% Ks molecule) than in the other nephelinite (20% - 25% Ks). Nepheline from the 0・2 GPa experiments has a relatively restricted compositional range, with an average composition of Ne77-74Ks19-2Qz3-4. Fresh nepheline shows a very constant composition close to Ne77Ks19Qz3, whereas in type nepheline, it has a lower proportion of quartz.
3.1. Spinel
Spinels chemical compositions were calculated on the basis of three cations and the proportions of Fe3+ and Fe2+ were determined on the basis of charge balance [30] . The high Al2O3 content indicates the low silicate activity [31] . Spinel is relatively poor in Cr and rich in Al. Al2O3 content varies from 58 to 59 wt%, MgO (18 - 19 wt%) and FeO (17 - 18wt%). This indicates that they were crystallized from an alkaline magma. Spinel type is pleonaste (Figure 6) and it is similar to the spinel which is included in clinopyroxenes at Oldoinyo Lengai, Tanzanie [4] .
![]()
Figure 6. Ternary plot of spinel diagram [32] .
3.2. Melilite
The minerals of the melilite group consist mainly of the solid-solution series between åkermanite (Ca2MgSi2O7), gehlenite (Ca2Al2SiO7) and ferro-akermanite (Ca2Fe2+Si2O7). The most melilite however contain appreciable amounts of Na replacing Ca as well as Fe2+ replacing Mg [28] . The melilite has been found only as microphenocrysts in melilite nephelinite and rare olivine nephelinite. They are iron and soduim rich (Fe2O3 6 to ~9wt%; Na2O 5 - 6 wt%). Melilite containing up to 6.0 wt% SrO. The compositional variations are slightest fairly that melilite in small olivine melilitite lavas flows on the floor of the rift valley to the east of Oldoinyo Lengai [25] . Ternary Diagrams [33] shows that composition corresponds to intermediate members of the ferro-akermmanite and Gehlenite.
4. Whole-Rock Chemistry
The variations observed in the major element composition (Table 1) could be partly explained by fractional crystallization processes. These variations are consistent with fractional processes dominated by removal of clinopyroxene, Ti-magnetite. Compared with other member nephelinite rock of the Oldoinyo Lengai silicate lava suite [34] , the late nephelinites have the lower SiO2 and Al2O3 contenent but the highest total iron, Cl and F concentrations. The nephelinite rocks are lower in the pneumotophile elements
concentration (Cl, Na, Fe, Mn, P, and Ti). The low MgO (<2 wt%), Ni (<15 ppm) and Cr (<12 ppm) concentrations indicate that these are evolved lavas. It also has Al2O3 concentrations similar to the other late nephelinite and despite the presence of Fe-rich rims on nepheline, sodalite and pyroxene phenocrysts.
4.1. Major Element
On the Harker diagram (Figure 7) with increasing MgO contents, TiO2, FeO and CaO contents generally increase and CaO/Al2O3 ratios are relatively constant at MgO < 6 wt% indicating olivine as the dominant crystallizing phase. Slightly positive correlations between MgO vs FeO, MgO vs CaO and TiO2 a negative correlation between MgO is observed at 5.5 wt% MgO. An interesting phenomenon is that when MgO contents are between 5.5 and 10.0 wt%, Al2O3 contents show a negative correlation with MgO whereas P2O5 contents exhibit a slightly positive correlation with MgO. It is possible that the high Al2O3 (≥19 wt%) contents of the more SiO2-rich, MgO-poor samples could be result of a process superimposed upon simple fractional crystallization.
![]()
Figure 7. Major element contents and their ratios against MgO variation diagrams for bulk rocks: TiO2 versus MgO, Al2O3 versus MgO, CaO versus MgO, Na2O versus MgO.
4.2. Trace Element Whole-Rock Compositions
Trace element analyses of the nephelinite from Etinde massif are reported in Table 3 and illustrated in primitive mantle-normalized diagrams (Figure 8).They are enriched in large ion lithophile elements (LILE, Rb, Ba, and Sr) and depleted in Ti (high-field strength elements, HFSE). They are also depleted in heavy rare earth elements (HREE) and enriched in light rare earth elements (LREE) relative to HREE. These trace element distribution patterns are similar to that of Oldoinyo Lengai nephelinite and the alkaline lavas of Cameroon Hot Line [1] . The trace element patterns of olivine nephelinite, melilitite and perovskite nephelinite are very similar (parallel) and characterized by a weak slightly positive Zr anomaly (Figure 8). The primitive mantle normalized trace element patterns of the nephelinite show negative P, K, and Ti anomaly. In detail, however, the
![]()
Figure 8. Multi-element diagrams normalized trace element and rare element plot for representative rocks; normalization values are from [42] .
![]()
Table 3. Whole rock chemical composition of the nephelinitic lavas representative from mount etinde analysis.
negative Nb anomaly is more significant for Etinde. They also show small Zr and Y positive anomalies where as small negative anomalies for these two elements are observed in the olivine nephelinite.
Nephelinite rocks has lower overall REE abundances compared with the haüynophyre, exhibiting different concave up REE pattern with steeper LREE part (La/Sm) = 18 compared with 4 - 5 in nephelinite. The trace element profile resembles that of nepheline from the Tanzania alkaline complex [4] . The total REE concentration and the slope of primitive normalized distribution patterns (La/Sm) ratios increase with differentiation. None of the samples analyzed for REE shows a significant Eu anomaly and this suggest no fractionation of plagioclase.
4.3. Mantle Source and Geodynamic Implications
Primitive mantle-normalized trace-element patterns of Etinde samples (Figure 8 and Table 3 for values) show sub-parallel shapes. The trace elements display a limited range of variations, indicating little influence of fractional crystallization differentiation. The samples nephelinite shows a negative P and Ti anomaly but no anomaly in Zr. This may indicate that apatite may be a residual phase but no ilmenite. The ranges in normalized La abundances and rather constant Yb abundances are consistent with melting occurring in garnet stability field. Nephelinite and melilitite have similar and parallel trace and REE normalized patterns. Primitive mantle-normalized trace-element compositions of the nephelinite rocks display a typical OIB signature with an enrichment of strongly incompatible element contents (Rb, Ba, Sr, Th). An important characteristic of the trace element enrichment patterns of the nephelinite, melilitite and haüynophyre is that the LILEs (Ba, Th) are at about the same level or slightly lower than the La (Figures 8(a)-(c)). The depletions in Cr and Ni and enrichments in incompatible elements such as Ba, Sr, Rb, Zr and Nb reflect olivine, pyroxene and spinel crystallization. Negative K and Rb anomalies accompanied by distinctive positive peaks of Ba and Nb are characteristic of basaltic rocks of anorogenic affinity of the Central European Volcanic Province [35] . The negative K and Rb anomalies accompanied by high and variable K/Rb and Zr/Hf ratios are typical of Bioko basaltic lavas and the Mount Cameroon [36] . These trace element features are believed to result from melting of mantle sources initially slightly depleted with metasomatized by slab-derived, H2O-rich phases segregating mobile from non mobile elements.
Geochemical characteristics observed on the nephelinite lavas are similar or identical of the Mount Cameroon lavas [37] [38] and [39] . The whole-rock atomic Mg/(Mg + Fe) ratio is practically identical to those of the basaltic lavas of the Cameroon Hot Line. The mineral assemblage of nephelinite could result from the basaltic magma by the pneumatolytic processes (reaction 1 and 2) and metasomatic processes (reaction 3) in agreement of [40] , [41] respectively by the reaction:
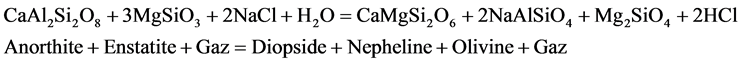 (1)
(1)
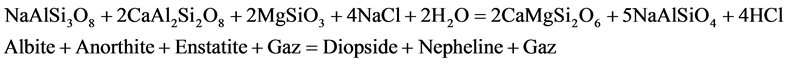 (2)
(2)
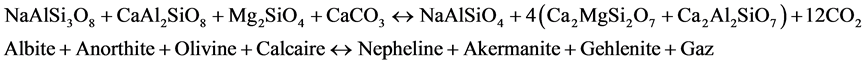 (3)
(3)
5. Discussion
The nephelinite rocks described here are insignificant volume when compared to the basaltic rocks of Mont Cameroon, but probably abundant ultramafic rocks of the Cameroon Hot Line. The interstitial mineral reveals ambiguous textural position. Although forming the analcime, there is ample textural evidence that it has replaced zeolite. This suggests that it is late magmatic origin. The oxygen fugacity lower and silica activity evolution at the late-magmatic and metasomatic process of evolution in the nephelinite could be constrained from mineral equilibria of the Equations (1), (2) and (3). The nephelinites have high Ba and Zr, and to a lesser degree high K. The Ba/Zr (1.4) and Ba/Nb ratios are relatively high compared to some nephelinite (Ba/Zr < l, Ba/Nb > 3) which suggests either amphibole was low in the mantle source [43] , or that its contribution to the magma became diluted through the suspected mantle fractionation. Early nepheline in the nephelinites is sodic (ne72-77), and compositions suggest crystallization around 980˚C, based on experimental isotherms [44] . Estimates for crystallization temperatures from nepheline, however, may be minimum values due to potential exchanges of alkalis and aqueous vapour that reset them to lower kls and qtz and so apparently lower temperatures [45] . Clinopyroxenes in the nephelinite rocks cumulate and assemblages mostly show relatively lower AlIV/AlVI ratios (0.9). Such ratios typically indicate low-pressure igneous crystallisation, rather than mantle and lower crust pressures [46] . Mass balance calculations performed with the olivine nephelinite give better fits (1 < Σr2 < 2).
6. Conclusions
Mineralogical, geochemical and trace elements are reported for the main nephelinite rocks on the Mount Etinde. The major and trace element composition of the host alkaline nephelinite suggest that fractional crystallization is not the only process involved in the petrogenesis of the nephelinitic lavas. The variation observed in their major and trace element position could be partly explained by fractional crystallization processes. The negative P anomaly may indicate that apatite may be a residual phase. These variations are consistent with crystallization processes dominated by removal of clinopyroxene ± olivine and nepheline by low aSiO2 and low fO2. The residual minerals in their source are mainly amphibole and garnet, possibly subordinate orthopyroxene (Equation (1)). The large variations documented by the isotopic data suggest the presence of heterogeneous mantle involving HIMU and EMI component [8] (Fitton, 1984). The principal mantle components invoke geochemical characteristics of magma source hybride nature for the Mount Etinde rocks, which are responsible for the main isotopic signature (87Sr/86Sr ranging between 0.7033 - 0.7030 and 143Nd/144Nd ranging between 0.512793 - 0.512891). All these suggest that the nephelinites were derived from a depleted mantle source rather than a peridotite source.
The high Zr/Hf (113 - 163) observed in the nephelinite lavas indicated the assimilation of carbonatic liquid near the magma chamber after the pneumatolytic processes of the basaltic magmas origin in agreement by [40] Rittmann (1963). Equations (1), (2) and (3) show that nephelinites are derived from the basaltic lavas composition. The volatile rich nature of Etinde lavas and their high abundance in incompatible trace elements require the presence of metasomatized mantle and thus refertilization of the mantle source before the partial melting event. The formation of rare, late mineral phases in the nephelinites is also attributed to late-magmatic and/or subsolidus hydrothermal processes.
Acknowledgements
We are grateful to the following individuals who have greatly aided this study.