Determination of Lanthanoids in Seawater by Inductively Coupled Plasma Mass Spectrometry after Pre-Concentration with a Chelating Resin Disk ()
1. Introduction
Lanthanoids (Ln’s; 57La-71Lu) are elements contained in high level radioactive wastes as fission products, and in addition, they are useful natural analogues for some transuranic (TRU) elements leaked into the natural environments [1] . Thus, Ln’s have been and are used in studies on the geological disposal of radioactive wastes as analogues for TRU elements [2] [3] . Grasping the behaviors of Ln’s in nature results in better understanding of those of TRU elements in nature. Ln’s are also often used as tracers in studies on natural environments because of their similar but consecutively changing ato- mic and ionic properties and frequently observed anomalous behaviors of cerium (Ce) and europium (Eu). Their concentrations in natural environments are, however, usually very low especially in aqueous environments. For instance, their concentrations in seawater are reported to be on the order of ng·kg−1 or lower [4] . Precise and reliable analytical techniques are thus indispensable. Previous investigations of Ln’s have largely been based on determinations by isotope dilution thermal ionization mass spectrometry (ID-TIMS). This method is precise, but sample preparation is quite tedious and time consuming, and the largest drawback of the method is that it cannot be applied to mono-isotopic elements. In earlier studies, De Baar et al. [5] - [7] employed neutron activation analysis (NAA) for the determination of Ln’s including the mono-isotopic ones. In our previous papers [8] - [10] , we applied NAA to the determination of Ln’s and thorium (Th) and uranium (U) in seawater- and brine-related substances. Unfortunately, the NAA method does not seem to be precise enough for a detailed analysis of geochemical behavior across the Ln’s. Shabani et al. [11] developed a method based on inductively coupled plasma mass spectrometry (ICP-MS). Nowadays, ICP-MS and ICP atomic emission spectroscopy (ICP-AES) seem the most commonly used method for the determination of trace Ln’s because of their capability of rapid multi-element detection over a wide concentration range with relatively low detection limits.
Although ICP-MS and ICP-AES are a powerful method for the determination of Ln’s in seawater and its related substances, their application to the determination of Ln’s at very low concentration level is limited because high salinity can cause matrix effects and the high concentration of barium (Ba) relative to Ln’s interferes the determinations of samarium (Sm) and Eu by forming their isobaric BaO+ and BaOH+ ions. Therefore, separation of matrix components and pre-concentration of Ln’s are a prerequisite. A variety of pre-concentration techniques have been adopted, which include co-precipi- tation, ion exchange, liquid-liquid extraction and solid-phase extraction (SPE). Among them, SPE based on the column chromatographic technique seems to be most frequently used. Such solid sorbents as chelating resin [12] [13] , chelating fiber [14] and carbon nanotubes [15] [16] have been used as column packing materials.
Chelating resin disks may be an alternative to chromatographic columns packed with a sorbent. For the determination of Ln’s in seawater by ICP-MS, we successfully used a chelating resin disk for separation of matrix components and pre-concentration of Ln’s. In this paper, we report the determination of Ln’s in seawater by ICP-MS after pre- treatment using a chelating resin disk, in which a combination of the internal standard method and the standard addition method is also included.
2. Experimental
2.1. Chelating Resin Disk and Chemicals, and Equipment
The chelating resin disk (CD) used was the 3M Company EmporeTM Chelating extraction disk for environmental analysis with the diameter of 47 mm and with the iminodiacetic acid component as the functional group. It was immersed in 3 mol·dm−3 HNO3 solution overnight prior to the application to separation of matrix components and pre-concentration of Ln’s. Water with the specific resistance of 18.2 MΩ・cm or higher at 25˚C (Milli-Q water) used throughout was produced with a Millipore water purification system. Stock standard solutions of Ln’s and indium (In) used as the internal standard substance and multielement standard solutions were purchased from Wako Pure Chemical Industries, Ltd. Working standard solutions were prepared by appropriate dilution and mixing of the stock standard solutions and addition of nitric acid (HNO3). All other chemicals used were of analytical grade or better. The seawater sample used was kindly donated by Nihonkaisui Co. Ltd. It was collected off the coast of Onahama, Fukushima, Japan in 2009, and used after sand filtration.
The ICP-MS instrument used was a Seiko Instrument Model SPQ9000 ICP mass spectrometer. The operating conditions are summarized in Table 1. The value of pH of solutions was measured with a Horiba twin pH B-212 pH meter. The glass filtration apparatus used was a product of Toyo Roshi Kaisha Ltd.
2.2. Preliminary Experiments
In advance of treating actual seawater, we first checked the performance of the CD using a sample solution containing lanthanum (La), gadolinium (Gd) and lutetium (Lu). La, Gd and Lu were intended to be the representatives of the light, middle and heavy Ln’s, respectively. Since the Ln’s are mainly dissolved in seawater as carbonate complexes like 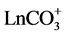 except Ce which forms Ce(OH)4 in seawater [4] , the sample solu-
except Ce which forms Ce(OH)4 in seawater [4] , the sample solu-
![]()
Table 1. Operating conditions for ICP-MS.
a: 142Ce interference was corrected with the ratio of 142Ce/140Ce.
tion for the preliminary experiments was prepared from 1 dm3 of 0.1 mol·dm3 sodium hydrogen carbonate (NaHCO3) solution according to the pre-treatment procedures for separation of matrix components and pre-concentration of Ln’s in aqueous solutions with the CD [17] . The preparation procedure of the sample solution was as follows. The pH of the 0.1 mol·dm3 NaHCO3 solution was first adjusted to <2 with HNO3. 5 mol· dm3 ammonium acetate (CH3COONH4) solution was then added to this pH-adjusted solution so that the concentration of CH3COONH4 became 0.1 mol·dm3, and the pH of the resultant solution was adjusted to 5 with HNO3 and ammonia water (NH4OH). Finally, a solution containing La, Gd and Lu was added to this solution to obtain the sample solution for the preliminary experiments. The contents of La, Gd and Lu in the sample solution were 49.5 ng, 50.0 ng and 49.5 ng, respectively.
The conditioning of the CD was carried out by referring to that of Maruta et al. [17] and Takaku et al. [18] . After set on a filter holder of the filtration equipment, the CD was wetted with 20 cm3 of Milli-Q water, washed with 20 cm3 of 3 mol·dm3 HNO3 and finally washed with 50 cm3 of Milli-Q water twice under suction. The chemical form of its functional group was then changed to the ammonium (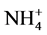 ) form with 50 cm3 of 0.1 mol·dm3 CH3COONH4, and the CD in the
) form with 50 cm3 of 0.1 mol·dm3 CH3COONH4, and the CD in the 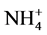 form was washed with a small amount of Milli-Q water. The CD thus conditioned was ready for use.
form was washed with a small amount of Milli-Q water. The CD thus conditioned was ready for use.
The whole volume of the above mentioned sample solution for the preliminary experiments was passed through the conditioned CD. After the CD was washed with 100 cm3 of 0.25 mol·dm3 CH3COONH4 solution, the Ln’s (La, Gd, Lu) adsorbed on the CD was eluted out with 35 cm3 of 2 mol·dm3 HNO3 solution. The effluent containing the Ln’s was evaporated to dryness on a hot plate, and the evaporation residue was dissolved with HNO3 solution. The volume of the solution was adjusted to 50 cm3 after 5.00 cm3 of an aqueous solution containing 100 μg·dm3 In used as the internal standard and 2 mol·dm3 HNO3 solution, whose volume was determined so that the final HNO3 concentration became 0.9 mol·dm3, were added. The solution prepared in this way was subjected to the ICP-MS. The concentration factor was thus 20.
2.3. Determination of Concentrations of Ln’s in Seawater
2.3.1. Determination by the Internal Standard Method after Pre-Concentration with the CD
The procedure for pre-treatment with CD was similar to that adopted in the preliminary experiments. The pH of the 4 dm3 aliquot of the seawater sample was first adjusted to <2. After its CH3COONH4 concentration was adjusted to 0.1 mol·dm3 by adding an appropriate volume of the 5 mol·dm3 CH3COONH4 solution, the pH was adjusted to 5 with HNO3 or NH4OH. The pH- and CH3COONH4 concentration-adjusted aliquot was passed through the CD. After the CD was washed with 100 cm3 of 0.25 mol·dm3 CH3COONH4 solution for the removal of alkali earth metals [18] , Ln’s adsorbed on the CD was eluted out with 35 cm3 of 2 mol·dm3 HNO3 solution. The effluent containing Ln’s was evaporated to dryness on the hot plate, and the evaporation residue was dissolved with HNO3 solution. The volume of the solution was adjusted to 20 cm3 after 2.00 cm3 of 100 μg·dm3 In solution and a certain volume of 2 mol·dm3 HNO3 solution
were added. The final solution whose HNO3 concentration was 0.6 mol·dm3 was subjected to the ICP-MS. The concentration factor in this case was 200. The concentrations of Ln’s in the final solution were calculated by the standard curve method incorporating the internal standard method using In as the internal standard. The standard curve for a Ln was obtained from the ratios of the counting rates of the Ln to those of In in the standard solutions, and the concentration of the Ln in the final solution, cLn, was calculated by interpolation on the curve. The concentration of the Ln in the seawater sample, cLn,A, in ng·dm3 is then given as Equation (1),
 (1)
(1)
The analytical procedure in this subsection is hereafter referred to as Procedure A.
2.3.2. Determination by Combination of Internal Standard and Standard Addition Methods after Pre-Concentration with the CD
The concentrations of Ln’s in the seawater sample were also determined using the combination of the internal standard and standard addition methods. This analytical procedure is hereafter referred to as Procedure B. The concentration of a Ln determined by Procedure B should be equal to that determined by Procedure A within experimental uncertainties if the Ln is completely trapped on the CD in the pre-concentra- tion treatment.
Known amounts of Ln’s were added to a 4 dm3 aliquot of the seawater sample. Those amounts were determined based on the analytical results obtained by Procedure A and are listed in Table 2. The Ln’s-added aliquot was subjected to the same treatment as the one in the previous subsection and the concentrations of Ln’s were determined by the internal standard technique. Assuming that the recoveries of a Ln from the aliquot and the Ln’s-added aliquot are the same in the pre-concentration process, the concentration of a Ln, cLn, B, in ng·dm3 in the seawater sample was obtained by Procedure B as Equation (2),
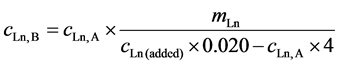 (2)
(2)
where mLn is the amount of the added Ln in ng and cLn (added) is the concentration of the Ln in ng·dm3 in the final solution of the Ln’s-added seawater sample.
3. Results and Discussion
3.1. Preliminary Experiments
Three similar experiments were conducted. The mean recovery was 107% ± 8%, 105% ± 7% and 96% ± 1% (± representing the 1σ) for La, Gd and Lu, respectively. This result shows that the CD trapped the Ln’s completely and they were completely eluted out with 2 mol·dm3 HNO3 solution after surviving the wash with 0.25 mol·dm3 CH3COONH4 solution within experimental uncertainties.
![]()
Table 2. Amounts of Ln’s added to 4 dm3 of seawater sample in standard addition method.
3.2. Concentrations of Ln’s in Seawater
The analytical results are summarized in Table 3. Ln’s on the order of ng·dm3 were found both by Procedures A and B. The standard deviations in Procedure B appear to be higher than those in Procedure A due to propagation of errors of cLn, A, cLn (added) and mLn. Procedure B yielded a higher concentration for every Ln except Eu and Tb than Procedure A, which strongly indicates that the CD adsorbed only a part of dissolved Ln. Although the preliminary experiments showed that the CD did adsorb all the amounts of the Ln’s in the sample solution, it is different from seawater in many ways. Seawater contains practically all naturally occurring elements, and the procedure using the CD can capture most multivalent metal ions except alkali earth metals. Although the decreasing order of the selectivity for trivalent ions on the imminodiacetic acid resin is reported as Fe3+ > La3+ > Al3+ [19] , many of divalent and trivalent metal ions are higher in concentration in seawater than Ln’s. It is thus reasonable that the CD could take up only a part of a Ln dissolved in 4 dm3 of the seawater sample. Consequently, the analytical results by Procedure B are considered to be more reliable than those by Procedure A.
In the standard addition method, the amount of the element to be added to the sample is important. In this study, the amounts of Ln’s added to the seawater sample in Procedure B were determined based on the results by Procedure A; that is, they mimicked those estimated from the concentrations in the seawater sample determined by Procedure A.
The recovery of a Ln in%, rLn, from seawater with the CD is calculable by Equation (3) and the results are summarized in Table 4.
 (3)
(3)
It varies between 41% to 100% with the simple arithmetic average of 76%. The recovery of neodymium (Nd) and Sm seems somewhat too low. The average is 81% if the Nd and Sm data are omitted.
![]()
Table 3. Concentrations of Ln’s in seawater.
a: Standard deviation (5 measurements).
![]()
Table 4. Recoveries of Ln’s from seawater with CD.
![]()
Figure 1. PAAS-normalized patterns of Ln’s in seawater.
Since there are no certified values of the concentrations of the Ln’s in the present seawater sample, the actual reliability of the data obtained by Procedure B is unknown. One way to check it may be to draw the Ln abundance pattern and compared it with those found in literature. The Ln abundance pattern obtained by normalization with the contents of the Ln’s in post-Archean average Australian shale (PAAS) [20] is shown in Figure 1 together with those by some other researchers [6] [18] [21] [22] . Our pattern is similar to the others as a whole. The negative Ce and positive Gd anomalies observed in our pattern seem to be reasonable. The negative Ce anomaly is commonly found in Ln abundance patterns of seawater due to the preferential scavenging of Ce(IV) with suspended matters like hydrous iron oxides. The determinant of the Gd anomaly in Ln abundance patterns of seawater is rather complicated. In the present case, it might be caused by the presence of anthropogenic organic complexes of Gd like Gd-DTPA used for a medical diagnosis, since the seawater sample used in this study was collected near the shore with moderate human activity. Such anthropogenic Gd anomalies in river waters and coastal seawaters were reported in several literatures [21] [23] - [25] . The alternative possible cause of the Gd anomaly is the differences in complexation behavior between Gd and its neighbors, Eu and Tb, in seawaters [6] [7] . Compared to the other determined Ln’s, Nd and Sm are obviously overestimated in our experiments, which must reflect the too low recoveries of Nd and Sm.
4. Conclusions
To summarize the present study, we make the following statements.
The chelating resin disk (3M EmporeTM chelating resin disk) is effective for the concentration of Ln’s from seawater. At the concentration factor of 200, the recovery of the Ln’s with the disk was 70% or better for the majority of the Ln’s.
In analyzing Ln’s in seawater by ICP-MS, the combination of the internal standard and standard addition methods was adopted. As a result, Ln’s on the order of ng·dm3 were found, which seems reasonable by the comparison with similar data in literature. The Ln abundance pattern shows the positive Sm and Gd anomalies and the negative Ce anomaly. Among them, the Sm anomaly is probably a fake ascribable to its too low recovery in the pre-concentration process with the chelating disk. Thus, a further optimization of the conditions for the pre-concentration of the Ln’s with the chelating disk is required. The incorporation of the standard addition method is indispensable to determine the concentration of Ln’s in seawater.