Co Complexes as a Corrosion Inhibitor for 316 L Stainless Steel in H2SO4 Solution ()
1. Introduction
In the last decade, various hetero atom substances have been studied extensively, among them the study of a good number of Schiff bases [1] . Furthermore, nitrogen and oxygen containing ligands and their complexes have a number of potentional technological applications [2] - [5] . Inhibitor is a keyword in the case of corrosion prevention by changing the chemistry of corrosive media. The use of inhibitors is one of the most practical methods for protection against corrosion especially in acidic conditions [6] [7] . Schiff base, an organic compound having general formula R-C=N-R' where R and R' are aryl, alkyl or cycloalkyl or heterocyclic groups formed by the condensation of an amine and a carbonyl group, is a potential inhibitor for different metallic surfaces. Several Schiff bases had been reported as effective corrosion inhibitors for carbon steel in HCl media [8] - [10] . The existing data show that these inhibitors act by adsorption on the metal/solution interface. However, studies about metal complexes as corrosion inhibitor for steels in acid solution appeared in the literature are extremely limited [11] [12] . Abdel-Gaber et al. [13] proposed corrosion inhibition through a bulky Co (III) Schiff base complex molecule could cover more than one active site, where carbon steel was immersed in H2SO4 solution. In this study, corrosion behavior of 316 L stainless steel in different concentration of Co complex as an inhibitor from 50 ppm to 200 ppm was evaluated using potentiodynamic polarization tests and impedance techniques.
In order to contribute to the scarcity of information in the literature on the corrosion inhibitory effects of complexes and their respective ligands, and to elucidate a possible mechanism for the corrosion inhibition of ligands and their respective complexes, the present work is aimed at studying the corrosion inhibitory properties cobalt complex on 316 L stainless steel in H2SO4 at different concentration.
2. Experimental
2.1. Materials
The chemical structure of synthesized Schiff-base Co complex has shown in Figure 1. Prior to all measurements, chemical composition of 316 L stainless steel specimens (in wt%) 0.03 C, 0.75 Si, 2 Mn, 0.045 P, 0.03 S, 2.3 Mo, 10.8 Ni and balance Fe were abraded successively with fine grade emery papers (600 - 1800 grade).
2.2. Methods
The specimens were washed thoroughly with double distilled water and finally degreased with acetone and dried at room temperature. For polarization and electrochemical impedance studies, the metal was embedded in epoxy resin, to expose a geometrical surface area of 1 cm2 to the electrolyte. The aggressive solution 0.1 M H2SO4 was prepared by dilution of analytical grade H2SO4 with double distilled water and all experiments were carried out in unstirred solutions.
In order to investigate the electrochemical corrosion behavior of samples, Potentiodynamic polarization and Electrochemical impedance measurements (EIS) studies were carried out using an electrochemical unit (EG&G 2273). A three electrode setup
![]()
Figure 1. Chemical structure of Co complexes.
was employed using a graphite of convenient area as counter electrode and a saturated calomel electrode (SCE) as reference electrode. The working electrode satinless steel (7.5 cm long stem) with the exposed surface of 1.0 cm2 was immersed into aggressive solutions with and without inhibitor and the open circuit potential was measured after 30 min, (the stable potential time).
EIS measurements were performed at corrosion potentials, Ecorr, over a frequency range of 100 kHz to 10 mHz with an AC signal amplitude perturbation of 10 mV peak to peak. Potentiodynamic polarization studies were performed with a scan rate of 1 mVs−1 in the potential range from 250 mV below the corrosion potential to 250 mV above the corrosion potential. All potentials were recorded with respect to the SCE. All measurements were done at 25˚C ± 0.1˚C. The specimens for surface morphological examination were rinsed quickly with distilled water and dried. The SEM analysis was performed on a scanning electron microscope (model LEO 1400VP).
3. Results and Discussion
3.1. Potentiodynamic Polarization Measurements
Polarization curves of 316 L SS at various concentrations of inhibitor in H2SO4 solution is shown in Figure 2(a). The respective electrochemical parameters including corrosion current density (icorr), corrosion potential (Ecorr), cathodic Tafel slope (βc) and anodic Tafel slope (βa) are listed in Table 1. It shows that value of Ecorr obtained in H2SO4 solution is shifted in the noble (positive) direction. This behavior indicates that this compound is mainly adsorbed on the anodic sites of the surface. The degree of surface coverage (θ) and the percentage of inhibition efficiency (IE%) were calculated using the following equations [14] :
 (1)
(1)
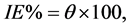 (2)
(2)
where  and
and 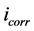 are corrosion current densities in the absence and presence of inhibitors, respectively. Comparison of curves, showed that with respect to the blank, increasing the concentration of Co complex gave rise to a consistent increase in the cathodic current density and addition of Co complex up to 200 ppm does not modify the mechanism of this process [15] . Co complex acts as a mixed-type inhibitor with predominant effect on the anodic reaction. Co complex at the lowest examined concentration (50 ppm) inhibits the anodic reaction (Fe dissolution) and accelerates the cathodic reaction (hydrogen evolution). Further addition of Co complex results in displacement of the anodic branches to low corrosion current density, while the cathodic branch remains approximately unaffected.
are corrosion current densities in the absence and presence of inhibitors, respectively. Comparison of curves, showed that with respect to the blank, increasing the concentration of Co complex gave rise to a consistent increase in the cathodic current density and addition of Co complex up to 200 ppm does not modify the mechanism of this process [15] . Co complex acts as a mixed-type inhibitor with predominant effect on the anodic reaction. Co complex at the lowest examined concentration (50 ppm) inhibits the anodic reaction (Fe dissolution) and accelerates the cathodic reaction (hydrogen evolution). Further addition of Co complex results in displacement of the anodic branches to low corrosion current density, while the cathodic branch remains approximately unaffected.
3.2. Electrochemical Impedance Measurements
Figure 2(b) shows Impedance measurements of 316 L stainless steel in 0.1 M H2SO4 solution alone and in the presence of various inhibitors concentrations. The recorded
![]()
Figure 2. (a) Polarization curves and (b) Nyquist plot of 316 L Stainless steel in 0.1 M H2SO4 in the absence and presence of inhibitor.
![]()
Table 1. Polariztion data of 316 L stainless steel in 0.1 H2SO4 at 25˚C ± 1˚C without and with differemmt concentrations of inhibitor.
EIS spectrum shows one depressed capacitive loop at higher frequency range (HF) followed by an inductive loop that is observed in the lower frequency region (LF). The equivalent circuit of EIS measurements shows in Figure 3. The intersection of the capacitive loop with the real axis represents the ohmic resistance of the corrosion product films and the solution enclosed between the working electrode and the reference electrode, Rs. Rct represents the charge-transfer resistance whose value is a measure of electron transfer across the surface and is inversely proportional to corrosion rate [16] . The constant phase element, CPE, is introduced in the circuit instead of a pure double layer capacitor to give a more accurate fit [17] . The electrochemical parameters obtained from fitting the recorded EIS data using equivalent circuit of Figure 3 are listed in Table 2. The inhibition efficiencies, IE%, of the tested inhibitors were calculated from the Rct values at different concentrations using the following equation [18] :
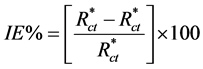 (3)
(3)
where  and Rct are the charge-transfer resistance values in the absence and presence of inhibitor, respectively. Increasing the concentration of Co complex increases the corresponding IE% values till Cinh =100 ppm, Figure 2(b) and Table 2. We can note from Figure 2(b) that the characteristic frequencies of the inductive loop diminish with increasing the magnitude of the electrode impedance whatever the inhibitor concentration is. In the high impedance Nyquist plots, the growing up inhibiting film delays the charge transfer processes to lower frequencies. The high impedance EIS curves were fitted to the one time constant equivalent circuit shown in Figure 3 where the induction loops shifted below the studied low frequency limit.
and Rct are the charge-transfer resistance values in the absence and presence of inhibitor, respectively. Increasing the concentration of Co complex increases the corresponding IE% values till Cinh =100 ppm, Figure 2(b) and Table 2. We can note from Figure 2(b) that the characteristic frequencies of the inductive loop diminish with increasing the magnitude of the electrode impedance whatever the inhibitor concentration is. In the high impedance Nyquist plots, the growing up inhibiting film delays the charge transfer processes to lower frequencies. The high impedance EIS curves were fitted to the one time constant equivalent circuit shown in Figure 3 where the induction loops shifted below the studied low frequency limit.
Inspection of the data reveals that its addition of the inhibitors increases the capacitive loop diameter of the Nyquist plots without affecting their characteristic features. This means that the inhibition action of this inhibitor is due to its adsorption on the metal surface without altering the corrosion mechanism. Impedance measurements
![]()
Figure 3. Equivalent circuits used to fit the EIS dats of 316 L stainless steel in 0.1 M H2SO4.
![]()
Table 2. Electrochemical impedance parameteres of 316 L stainless steel in 0.1 H2SO4 solution in the absence and presence varioud concentrations of inhibitor.
confirmed polarization tests results.
3.3. Adsorption Studies
The mechanism of corrosion inhibition may be explained on the basis of adsorption behavior. Figure 4 shows the dependence of θ as function of the logarithm of inhibitor concentration. The data fit straight lines of slopes more than unity indicating that this inhibitor is adsorbed according to Langmuir’s isotherm.
3.4. Surface Characterization
Surface morphology of the 316 L stainless steel samples with and without 100 ppm in-
![]()
Figure 4. Langmuir adsorption isotherm of Co complex in 0.1 H2SO4 solution at 25˚C.
![]()
Figure 5. SEM images of 316 L stainless steel after potentiodynamic polarization tests in 0.1 M H2SO4 (a) without and (b) with 100 ppm inhibitor.
hibitor after potentiodynamic polarization tests in 0.1 M H2SO4 solution shows in Figure 5(a) & Figure 5(b). The SEM photograph [Figure 5(a)] shows that the surface is highly damaged in the absence of the inhibitor while Figure 5(b) shows the formation of a film on the metal surface which may be responsible for the corrosion inhibition. It is well known that the first step in the inhibition of acid corrosion is the adsorption of inhibitor molecules onto the metal surface. Due to the electrostatic attraction, the cationic Co complex molecules are adsorbed (physical adsorption) and high inhibition effect is expected.
4. Conclusion
Co complex is a moderate inhibitor for 316 L stainless steel corrosion in H2SO4 solution. Electrochemical tests revealed that increasing the concentration of Co complex increases the corresponding IE% values till 100 ppm. Adsorption of Co complex obey Langmuir adsorption isotherm. Co complex acts as mixed type inhibitors with predominant effect on the anodic dissolution of iron.