Seasonal Quality Assessment of Agricultural Soils along the Bank of Tungan Kawo Dam, Kontagora, Nigeria ()
Received 30 May 2016; accepted 14 August 2016; published 17 August 2016

1. Introduction
Pollution of the environment is an important problem in human’s life that leads to a miserable condition. Environment usually affected include: air, soil, water and vegetations which have direct effect on human life. The soil being the repository of all pollutants is most affected because soil is used for vegetation of edible plants and plants are taken in as part of our food. Soil is a very important natural resource to man as it is a source of his life on this planet. Despite its importance, soil is often contaminated by human activities and this is reflected in the high horizontal and vertical variability brought about by the anthropogenic influence on soil formation and development [1] .
A variety of human activities including municipal waste disposal, industrial emissions, and agricultural practices have left their impacts on soils in the form of elevated and high level of toxicants especially the heavy metals [2] . The concentration of heavy metals in soil and their impact on ecosystems can be influenced by many factors such as the parent rock, climate and anthropogenic activities [3] . Among the pollutants that persist and accumulate in the soils include: inorganic toxic compounds. The soil is thus becoming increasingly polluted with chemicals and other pollutants which can reach the food chain, surface water or ground water and ultimately be ingested by man [4] .
In Northern Nigeria, fadama is a common agricultural practice mostly located within the banks around rivers, dams and other major water sources that stay throughout the year and are often used for irrigation activities covering but not limited to the irrigation of assorted vegetables and other root crops [5] - [7] . Fadama is a Hausa word meaning the seasonally flooded or floodable plains along major savannah rivers and/or depressions on the adjacent low terraces [8] . Fadama utilization has been a major feature of the agricultural, food, economic and demographic experience of the Nigerian dry belt. The rationale for resource utilization here hinges on the availability of valuable agricultural resources in a zone where rain fed agricultural prospects are poor due to the small and erratic nature of rainfall and endemicity of drought [9] . Of a particular threat to fadamaor lowland irrigation crops in dry land areas located along dams are effluents agricultural practice and households especially where there no industrial contributions which contaminate irrigation channels. Tungan Kawo dam irrigation scheme in Kontagora is not different from the foregone problems. From literature, no information is available on the quality assessment of the agricultural soils along the bank of Tungan Kawo dam, hence, imperative for this study. Therefore, the study aimed to elucidate the quality of the agricultural soil through the following objective; determination of physicochemical parameters, metal concentration based on their geochemical forms and confirmation of their risk to plants.
2. Materials and Methods
2.1. Study Area
The Tungan Kawo Dam is located State between latitude 10˚21"58.51˚N - 10˚23"28.50˚N of the equator and between longitude 5˚19"29.23˚E - 5˚20"59.23˚E in Tungan Kawo village, northwest of Kontagora, 7 km along Kontagora-Yauri road in Kontagora Local Government Area of Niger State. The dam has a catchments area of 143 km2 with a total storage capacity of 17.7 M Cubic meters, 20 m high and dam crest length of 1000 m. The dam was commission in May 1991. It is the largest source of water supply in Kontagora Township. The people of Tungan Kawo and its environs are predominantly farmers and have remained so for years. In this area, vegetables are irrigated with dam water and all kinds of available waste and polluted waters. Similarly, to enhance the yield of these vegetables, fertilizers and manures are occasionally added to the soil [10] .
2.2. Sampling
The farmland was divided into five sampling plots as shown in Figure 1 and soil samples were collected within the rooting depth (0 - 15 cm) during when crops are harvested across the seasons. A total of fifteen (15) samples were collected from the five (5) sampling plots with the aid of a stainless bottom grab. The content of the grab was emptied into a black polythene bag [11] at each location, labelled and taken to the laboratory for processing/pre-treatment and analysis. Each sampling site comprises of three (3) samples composites to form a core for the site. Samples were collected on a seasonal basis between February 2012 and September 2013 as follows:
Seasons
Cool and dry season: December-February
Hot and dry season: March-May
Warm and wet season: June-August
Warm and dry season: September-November
![]() Source: Adapted and Modified from Europa technologies Google Earth Image, 2010
Source: Adapted and Modified from Europa technologies Google Earth Image, 2010
Figure 1. Tungan Kawo dam showing sampling sites and vegetation areas.
2.3. Sample Pre-Treatment
Soil samples for metal analysis were allowed to air-dried. Dry soils were ground into fine powder and homogenized using an acid-washed clean mortar and pestle and sieved to give 200 μm particle size. They were then stored in desiccators to attain constant weight before being stored in air-tight plastic bottles. All metallic determination from soil samples was based on the fine particles obtained.
2.4. Sequential Extraction
Sequential extraction was carried out on the principle of selective extraction proposed by [12] . This method modified the conventional method developed by [13] . The modified method determines fractionation of heavy metals into six (6) geochemical fractions. 1.0 g of the homogenized sample was weighed into a conical flask and appropriate extractants were added and the pH of solution matrix was adjusted following a standard literature procedure according to [12] . The extract from the six stages of extractions were separately stored in a plastic vials prior to AAS analysis. The total concentrations of each metal were calculated as sum of the six fractions from the metal. Samples were analysed in triplicate. Sample treatment, extraction and subsequent analytical determinations were performed in a clean laboratory.
Quality control test was performed on soil and plant samples in order to validate the experimental procedures. This was done by spiking the pre-digested soil and plant samples with multielement metal standard solution (0.5 mgL−1 of Cd and Cr and 5 mgL−1 for Cu, Fe, Mn, Ni, Pb and Zn). The percentage recovery showed that 90% - 95% of the spiked metals were recovered, indication of reliability of the results obtained.
2.5. Method Validation
The efficiency of the digestion method was validated by a spiking experiment as described in the preceding chapter. The percentage recoveries of metals ranged from 99.0 - 108.0 in pepper and 90.0 - 100.0 in soils. The pattern of recovery efficiency in both pepper and soil were found to follow the decreasing order, Cd > Pb > Ni > Mn > Cu > Zn > Cr and Pb > Mn > Cd > Zn > Cu > Ni > Cr, respectively. However, high recoveries above 90% in this study could imply that the procedures used for treatment and analysis of sample is efficient.
2.6. Physicochemical Parameters
The pH and electrical conductivity of sediment samples was measured with sediment: water ratio 1:2 using pH and electrical conductivity meters as described by [14] , organic carbon was determined by the method of [15] , cation exchangeable capacity (CEC) was determined by [14] , particle size distribution was determined by the hydrometer method as described by [16] . Also, extractable nitrate (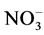 ), chloride (
), chloride (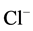 ), sulphate (
), sulphate (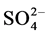 ) and available phosphate (
) and available phosphate (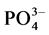 ) were determined by methods described by [17] .
) were determined by methods described by [17] .
3. Results and Discussion
3.1. Physicochemical Parameters
The results of variation in physicochemical parameters from the study and control area in the four seasons are presented in Tables 1-4. The results of the pH for study site range from 5.77 (site 5) - 7.17 (site 1), 6.40 (sites 2 and 3) - 6.62 (site 1), 9.00 (site 4) - 10.23 (site 2), and 7.37 (site 3) - 7.49 (site 2) for cool and dry season, hot and dry season, warm and wet season, and warm and dry season respectively. For the control site, the pH fraction was 7.50, 6.20, 6.20 and 6.50 in the same order of seasons. pH in CaCl2(aq) for the study site showed 5.10 (site 3) - 5.27 (site 1), 5.27 (site 1) - 5.50 (site 3), 6.20 (site 5) - 6.67 (site 2) and 6.30 (sites 1 and 4) - 6.50 (site 3) for cool and dry season, hot and dry season, warm and wet season, and warm and dry season respectively. Also, for the control site, the pH in CaCl2(aq) was found to be 6.50, 5.10, 5.10 and 5.10 across the seasons in the
![]()
Table 1. Physico-chemical parameters of soil samples during cool and dry season.
EC: Electrical Conductivity; OM: Organic Matter; TN: Total Nitrogen; AP: Available Phosphorus; S: Sulphur; Cl−: Chloride; CEC: Cation Exchange Capacity.
![]()
Table 2. Physico-chemical parameters of soil samples during hot and dry season.
EC: Electrical Conductivity; OM: Organic Matter; TN: Total Nitrogen; AP: Available Phosphorus; S: Sulphur; Cl−: Chloride; CEC: Cation Exchange Capacity.
![]()
Table 3. Physico-chemical parameters of soil samples during warm and wet season.
![]()
Table 4. Physico-chemical parameters of soil samples during warm and dry season.
EC: Electrical Conductivity; OM: Organic Matter; TN: Total Nitrogen; AP: Available Phosphorus; S: Sulphur; Cl−: Chloride; CEC: Cation Exchange Capacity.
same order as the study sites. Analysis of variation among the study sites across the seasons showed significant differences (p < 0.05). The varying sources of effluent discharge into the dam across the seasons could be a possible reason. Comparing the pH of study and control sites, pH at the control site is alkaline across the seasons.Within pH range of 5.8 - 6.5, acidity is present (apparently from hydroxyl-Al and organic functional groups, ordinarily hydronium, in amounts sufficient to affect acid-sensitive crops [18] ). This could imply high mobility of metals within these sites to other environmental compartment. For pH at 6.5 - 8.0 the soil is essentially fully base saturated, with large amounts of exchangeable Ca and Mg [19] . The pH values recorded in the study areas are similar to pH values 5.5 to 6.3 and 5.40 to 7.60 reported by [20] and [9] , respectively for some other Nigerian irrigated soils.
The electrical conductivity (EC) across the seasons, cool and dry, hot and dry, warm and wet, and warm and dry showed the range: 0.01 (site 3) - 1.31 (site 4), 0.05 (site 2 and 5) - 0.10 (site 1), 0.07 (site 2 and 4) - 0.30 (site 1), and 0.09 (site 3, 4 and 5) - 0.15 μS/cm (site 1) for the seasons respectively, 0.05, 0.10, 0.10 and 0.10 μS/cm were the EC values for the control site across the seasons in the same order. There were significant differences (p < 0.05) in EC among the various sites. Highest values of EC were recorded at site 4 during cool and dry season. Analysis of variation also indicated that mean values of EC did vary between and within seasons, and did show seasonal pattern. Generally, the values reported in this study are lower than 3 - 80 μS/cm reported in Bakori Dam irrigation soils [20] . By comparison, [21] classified ECE of soils (in dSm−1) as: non-saline <2; moderately saline 2 - 8; very saline 8 - 16; extremely saline >16. Across the seasons EC < 1 which indicated no acute problems with soil salinity.
Seasonal variation in percentage organic matter (OM) and the variations between sampling sites were observed. The OM varied between 0.52 (site 2) - 0.78 (site 1 and 4), 0.87 (site 3) - 1.60 (site 2), 1.86 (site 4) - 2.10 (site 1), and 0.97 (site 3) - 1.62% (site 2) for cool and dry season, hot and dry season, warm and wet season, and warm and dry season, respectively for study sites. However, 2.35, 1.14, 1.14 and 1.14 were observed for the control site across the seasons. Analysis of variation among the study sites across the seasons showed significant differences (p < 0.05). Higher OM content in control site compare to study site (cool and dry season) could be due decomposition of weed plants over a long period as compare to study area where these are not found. The range of OM in this study is lower than 2.04% to 2.20% reported by [9] . However, the values are typically low and characteristic of savanna soils because of rapid decomposition of plant and animal residues added to soil [22] . This implies that the soil organic matter contains humic materials with low complex functional groups, which have the ability to complex metals thereby retaining them in the topsoil [23] . The more organic matter is present in soil, the more functional groups available for complexation with the metals, hence, the more the retention [24] . The organic matter of soils in the present study indicated low retention of metals, thereby making them available for plant uptake.
The total nitrogen (TN) in the soil varied between 0.04 (site 2) - 0.08% (site 4) for cool and dry season, 0.05 (site 1) - 0.08% (site 2, 3 and 4) for hot and dry season, 0.07 (site 1) - 0.09% (site 3, 4 and 5) for warm and wet season, and 0.07 (site 1 and 5) - 0.09% (site 3) during warm and dry season. Across the seasons highest TN was recorded at site 3 (0.09%d) during warm and dry season. For the control site, TN (%) were observed to be 0.18, 0.06, 0.06 and 0.06 for cool and dry season, hot and dry season, warm and wet season, and warm and dry season, respectively. There were no significant differences (p > 0.05) in EC among the various sites. Analysis of variation also indicated that mean values of EC did vary between and within seasons, and did show seasonal pattern. Across the seasons, there is a general increase in TN in all sites, this suggest increase in content of nitrogen in the soils. The range of TN in this study is lower than the range of 0.5 to 21.9% reported in Nigeria [20] .
The concentration of nitrate (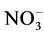 ) in the soil samples fluctuated between 0.01 mg/l (at all sites), 0.01 (site 2, 3, 4 and 5) - 0.02 mg/l (site 1), 1.05 (site 2) - 1.75 mg/l (site 1), and 1.11 (site 2, 3, 4 and 5) - 1.17 mg/l (site 1) during the cool and dry season, hot and dry season, warm and wet season, and warm and dry season, respectively. Also, across the season highest % OM was recorded for site 1 (1.75 mg/l) during warm and wet season. For control site,
) in the soil samples fluctuated between 0.01 mg/l (at all sites), 0.01 (site 2, 3, 4 and 5) - 0.02 mg/l (site 1), 1.05 (site 2) - 1.75 mg/l (site 1), and 1.11 (site 2, 3, 4 and 5) - 1.17 mg/l (site 1) during the cool and dry season, hot and dry season, warm and wet season, and warm and dry season, respectively. Also, across the season highest % OM was recorded for site 1 (1.75 mg/l) during warm and wet season. For control site, 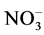 values were 0.01, 0.01, 1.02 and 0.01 mg/l for cool and dry, hot and dry, warm and wet, and warm and dry seasons, respectively. Analysis of variance among the various sampling sites showed significant differences (p < 0.05). Generally, highest concentrations of
values were 0.01, 0.01, 1.02 and 0.01 mg/l for cool and dry, hot and dry, warm and wet, and warm and dry seasons, respectively. Analysis of variance among the various sampling sites showed significant differences (p < 0.05). Generally, highest concentrations of 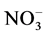 in soil were revealed during warm and dry season. Nitrate is among one of the macro nutrients necessary for plant growth [25] . The magnitude of nitrate in leaching will depend on the amount of nitrates in the soil, amount and time of rainfall, infiltration and percolation rates and also the water-holding capacity of the soil [26] . The amount of nitrate in the study area is higher than the control area, this suggest input of fertilizer used during irrigation responsible for the increase in the study sites. This also corroborate with the increase in the coefficient of nitrate during hot and dry, warm and wet, and warm and dry seasons. Also, from the results in the cool and dry season, there seems to be no clear trend in the level of nitrate across the sites. However, there is a gradual increase in nitrate across the sites down the warm and dry season. The values in this study is lower than range of 0.2 - 4.62 mg/L reported by [20] .
in soil were revealed during warm and dry season. Nitrate is among one of the macro nutrients necessary for plant growth [25] . The magnitude of nitrate in leaching will depend on the amount of nitrates in the soil, amount and time of rainfall, infiltration and percolation rates and also the water-holding capacity of the soil [26] . The amount of nitrate in the study area is higher than the control area, this suggest input of fertilizer used during irrigation responsible for the increase in the study sites. This also corroborate with the increase in the coefficient of nitrate during hot and dry, warm and wet, and warm and dry seasons. Also, from the results in the cool and dry season, there seems to be no clear trend in the level of nitrate across the sites. However, there is a gradual increase in nitrate across the sites down the warm and dry season. The values in this study is lower than range of 0.2 - 4.62 mg/L reported by [20] .
The available phosphorus (AP) across the seasons, cool and dry, hot and dry, warm and wet, and warm and dry showed the range: 7.81 (site 1) - 13.13 mg/L (site 2), 7.00 (site 2) - 7.17 mg/L (site 5), 7.77 (site 1) - 8.33 mg/L (site 4), and 6.63 (site 2) - 29.71 mg/L (site 1) for the seasons respectively, 136.40, 7.10, 5.17 and 5.20 mg/L were the AP values for the control site across the seasons in the same order. There were significant differences (p = 0.021) in AP among the various sites. Highest values of AP were recorded at site 4 during cool and dry season. Analysis of variation also indicated that values of AP vary (p < 0.05) between the seasons. Similar to nitrogen, phosphorus is an essential elements classified as macronutrient because of the relatively large amounts of phosphorus required by plants [25] . Phosphorus is among the nutrient added to the soils through the application of fertilizer, hence, comparison of AP between study and control sites showed that control site has high level of phosphorus, this suggest possible input of phosphate from other anthropogenic sources. Across the seasons, there is no clear trend in the level of AP in the sites, although highest range of 6.63 - 29.17 mg/100g was observed in warm and dry season. This could be related to strong positive correlation between the AP concentration and pH and organic matter. Similar relationship was also reported by [25] . However, the concentration of AP was higher than 0.3 - 0.6 mg/100g reported in related study in Nigeria [20] .
Sulphate (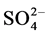 ) varied between 8.50 (site 1) - 15.70 mg/l (site 2) in cool and dry season, 9.60 (site 1) - 20.53 mg/l (site 2) in hot and dry season, 10.90 (site 5) - 20.53 mg/l (site 2) in warm and wet season, and 9.60 (site 1) - 11.53 mg/l (site 2) in warm and dry season. Highest concentration of sulphate was recorded in warm and wet season. Analysis of variation across the seasons showed significant difference (p < 0.05) in the values. For control site,
) varied between 8.50 (site 1) - 15.70 mg/l (site 2) in cool and dry season, 9.60 (site 1) - 20.53 mg/l (site 2) in hot and dry season, 10.90 (site 5) - 20.53 mg/l (site 2) in warm and wet season, and 9.60 (site 1) - 11.53 mg/l (site 2) in warm and dry season. Highest concentration of sulphate was recorded in warm and wet season. Analysis of variation across the seasons showed significant difference (p < 0.05) in the values. For control site, 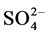 values were 15.07, 9.84, 9.84 and 9.70 mg/l for cool and dry, hot and dry, warm and wet, and warm and dry seasons, respectively. The profile of sulphate (
values were 15.07, 9.84, 9.84 and 9.70 mg/l for cool and dry, hot and dry, warm and wet, and warm and dry seasons, respectively. The profile of sulphate (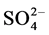 ) content of the soils of the study areas decreases in the warm and dry season (9.60 - 11.53 mg/l). The decrease in sulphate content of the soils could result from the parent materials, high leaching rates, crop removal and low level of atmospheric sulphur-bearing air [27] . Retention of sulphate in soils is highly dependent on pH and mineralogy and this is supported with strong positive correlation between
) content of the soils of the study areas decreases in the warm and dry season (9.60 - 11.53 mg/l). The decrease in sulphate content of the soils could result from the parent materials, high leaching rates, crop removal and low level of atmospheric sulphur-bearing air [27] . Retention of sulphate in soils is highly dependent on pH and mineralogy and this is supported with strong positive correlation between![]() , pH and organic matter. Also the fate of sulphate in the soil is influenced by many chemical, biological and physical factors and the ability of the soil to adsorb the sulphates occurs above a pH of 6.5 but adsorption increases as pH decreases [28] , this was not observed in this study. In comparison to other studies range of values across the seasons was lower than 13.7 - 94.4 mg/L reported in literature [20] . Fluctuation in amount of
, pH and organic matter. Also the fate of sulphate in the soil is influenced by many chemical, biological and physical factors and the ability of the soil to adsorb the sulphates occurs above a pH of 6.5 but adsorption increases as pH decreases [28] , this was not observed in this study. In comparison to other studies range of values across the seasons was lower than 13.7 - 94.4 mg/L reported in literature [20] . Fluctuation in amount of ![]() across the season may occur as a result of leaching, mineralization of organic sulphur and uptake by plants [25] .
across the season may occur as a result of leaching, mineralization of organic sulphur and uptake by plants [25] .
Chloride (Cl−) fluctuated between 0.25 (site 3 and 5) - 0.46 mg/l (site 1), 0.57 (site 3 and 4) - 0.80 mg/l (site 1), 0.90 (site 2) - 1.46 mg/l (site 3), and 0.57 (site 3 and 4) - 0.80 mg/l (site 1) in during the cool and dry season, hot and dry season, warm and wet season, and warm and dry season, respectively. Analysis of variance showed that there were significant differences (p < 0.05) in the values of chloride recorded among the various sampling sites across the seasons. Again there was no clear seasonal pattern observed. For control site, Cl− values were 2.23, 0.50, 0.40 and 0.50 mg/l for cool and dry, hot and dry, warm and wet, and warm and dry seasons, respectively. According to [25] , Cl− content of the soil is not an intrinsic property of the soil, but is a result of soil management, because of the its mobility in the soil and the fact that it moves with the water in the soil. This could explain the increase in the amount of Cl− during the warm and wet season (0.90 - 1.46 mg/l) with a slight decrease (0.57 - 0.80 mg/l) in warm and dry season―a season after rains. This occur as a result of the fact that Cl− is not adsorbed on the soil particles at neutral and alkaline pH values [29] which is the pH prevalence during the warm and wet, and warm and dry seasons. The Cl− concentrations in the control sites are higher than the study sites in cool and dry season, this could occur as a result of anthropogenic inputs. The results recorded in this study are lower compare to 1.2 - 40.8 mg/l reported in Bakori [20] .
CEC also varied between 5.17 (site 4) - 9.20 Cmolkg−1 (site 2), 7.83 (site 2) - 8.63 Cmolkg−1 (site 5), 8.90 (site 5) - 10.43 Cmolkg−1 (site 2), and 8.70 (site 5) - 8.83 (site 1) for cool and dry, hot and dry, warm and wet, and warm and dry seasons, respectively. Significantly, there were variations (p < 0.05) among sites across seasons with lowest mean value of CEC recorded in the Cool and Dry Season and the highest mean value recorded in the warm and wet season. For control site, CEC values were 10.13, 6.20, 5.17 and 5.50 Cmolkg−1 for cool and dry, hot and dry, warm and wet, and warm and dry seasons, respectively. Similar, to percentage organic matter content, highest CEC were obtained during warm and wet season across all sites. Analysis of variation showed significant differences (p < 0.05) in the value of CEC recorded among the sites across the seasons. Comparing the study and control sites, high values were obtained in study sites with exception in cool and dry season. The CEC of soils is related to the nature and quality of clay and organic matter. Hence, high % organic carbon from these sites could be responsible for the CEC. According to [9] , CEC of soil is more greatly influenced by organic matter than by the concentration of clays, hence CEC tends to be higher in the study sites than the control sites. For savanna soils dominated by low-activity clay and low organic matter, the CEC is quite low. According to [22] and Wild (1975), CEC of savanna soils is more greatly influenced by organic matter than by the concentration of clays, hence CEC tends to be higher in the studied soils.
Multivariate correlation analysis between various soil physicochemical parameters across the seasons is presented in Tables 5-8. Some of the parameters were found to bear statistically significant correlation with each
![]()
Table 5. Correlation matrix among metals in soils physicochemical parameters during cool and dry season.
**Correlation is significant at the 0.01 level (2-tailed). *Correlation is significant at the 0.05 level (2-tailed).
![]()
Table 6. Correlation matrix among metals in soils physicochemical parameters during hot and dry season.
**Correlation is significant at the 0.01 level (2-tailed). *Correlation is significant at the 0.05 level (2-tailed).
![]()
Table 7. Correlation matrix among metals in soils physicochemical parameters during wet and warm season.
**Correlation is significant at the 0.01 level (2-tailed). *Correlation is significant at the 0.05 level (2-tailed).
![]()
Table 8. Correlation matrix among metals in soils physicochemical parameters during September-November.
other indicating close association of the parameters. In the cool and dry season (Table 7), for example, data showed high significant positive correlation between the OM: pH, TN: pH and OM, AP: pH, OM and TN, Cl−: pH, OM, TN and AP, and CEC: pH, OM, AP, ![]() and Cl− (p < 0.05). On the contrary in hot and dry season (Table 8), significant positive correlations (p < 0.05) were observed between TN: pH,
and Cl− (p < 0.05). On the contrary in hot and dry season (Table 8), significant positive correlations (p < 0.05) were observed between TN: pH,![]() : pH, ECE,
: pH, ECE,![]() : OM, Cl−: pH and
: OM, Cl−: pH and![]() , and ECE: pH. Similarly, in warm and wet season (Table 9), significant positive correlations (p < 0.01) were observed between: OM: pH, TN: pH and OM,
, and ECE: pH. Similarly, in warm and wet season (Table 9), significant positive correlations (p < 0.01) were observed between: OM: pH, TN: pH and OM,![]() : ECE, OM, AP: pH, OM and TN,
: ECE, OM, AP: pH, OM and TN,![]() : pH, OM and AP, Cl−: pH, OM, TN and AP, and CEC: pH, OM, TN, AP,
: pH, OM and AP, Cl−: pH, OM, TN and AP, and CEC: pH, OM, TN, AP, ![]() and Cl‑. In warm and dry season (Table 10), similar relationships were observed between the parameters as recorded in cool and dry season with few exceptions. On the other hand, negative correlation was also observed between CEC: ECE (p < 0.01) in cool and dry season, ECE: pH and CEC, and TN: ECE in hot and dry season (p < 0.01). Significant negative correlations among the physicochemical parameters imply that increase in level of one of the parameter would increase the other.
and Cl‑. In warm and dry season (Table 10), similar relationships were observed between the parameters as recorded in cool and dry season with few exceptions. On the other hand, negative correlation was also observed between CEC: ECE (p < 0.01) in cool and dry season, ECE: pH and CEC, and TN: ECE in hot and dry season (p < 0.01). Significant negative correlations among the physicochemical parameters imply that increase in level of one of the parameter would increase the other.
3.2. Total Metal Concentration in Soil
The total metals concentration of namely Cd, Cr, Cu, Fe, Ni, Pb and Znin soils samples from the sites studied are presented in Tables 9-12. In each case the presented value is a mean observed in three determinations. Analysis of variance (ANOVA) revealed significant differences (p < 0.05) in the contents of all the heavy metals across seasons with exception of Cr and Zn. The concentrations across the seasons varied to a greater extent among the samples: 2.82 (site 4 and 5) - 2.86 (site 1), 1.00 (site 5) - 1.21 (site 1), 3.32 (site 4) - 3.38 (site 1) and 3.32 (site 1) - 4.31 mgkg−1 (site 5) for Cd, 52.87 (site 2) - 107.83 (site 5), 29.58 (site 5) - 32.57 (site 1), 24.44 (site 5) - 25.73 (site 1) and 27.94 (site 3) - 29.61 mgkg−1 (site 1) for Cr, 48.01 (site 4) - 48.65 (site 5), 2.22 (site 4) - 2.42 (site 2), 5.69 (site 5) - 5.81 (site 1) and 6.88 (site 2) - 7.40 mgkg−1 (site 5) for Cu, 699.74 (site 5) - 701.72 (site 1), 3156.82 (site 4) - 3162.18 (site 1), 3821.57 (site 3) - 7540.01 (site 4) and 3076.76 (site 4) - 3160.51 mgkg−1 (site 2) for Fe, 7.51 (site 4) - 7.65 (site 1), 23.82 (site 5) - 26.27 (site 1), 29.19 (site 4) - 29.56 (site 1)
![]()
Table 9. Concentration (mg/kg) of metals in soils during cool and dry season.
![]()
Table 10. Concentration (mg/kg) of metals in soils during hot and dry season.
![]()
Table 11. Concentration (mg/kg) of metals in soils during warm and wet season.
and 28.24 (site 3) ? 29.03 mgkg−1 (site 1) for Mn, and 136.39 (site 4) - 137.60 (site 2), 14.61 (site 3) - 17.29 (site 4), 17.05 (site 5) - 17.54 (site 1) and 16.96 (site 3 and 4) - 43.21 mgkg−1 (site 1)for Ni, 162.89 (site 2) - 164.45 (site 1), 12.16 (site 5) - 12.85 (site 1), 12.53 (site 4) - 12.76 (site 1) and 12.94 (site 3) - 13.74 mgkg−1 (site 1) for Pb, and 271.30 (site 5) - 273.32 (site 3), 225.15 (site 5) - 227.52 (site 1), 342.69 (site 5) - 344.47 (site 1) and 319.82 (site 4) - 321.41 mgkg−1 (site 1) for Zn in cool and dry, hot and dry, warm and wet, and warm and dry seasons, respectively.
Irrespective of sampling site, the distribution of total metals in the soil samples generally followed the order: Fe > Zn > Cr > Mn > Pb > Ni > Cu > Cd. Highest levels of Cd, Cr and Cu were found at site 5, while highest concentrations of Fe, Mn, Pb and Zn, and Ni were found at site 1 and 2, respectively. Also in all the sites, highest concentration of Cr, Cu, Ni and Pb was recorded in cool and dry season, Fe, Mn and Zn was recorded in warm and wet season, and Cd was recorded in warm and dry season.
Correlation analysis as shown in Tables 13-16 indicates significant positive correlation (p < 0.05) between Pb and Cd (cool and dry season), Cd: Cr and Zn, Cu: Zn and Fe, and Mn: Pb and Zn (hot and dry season), Cu: Cr, Cd: Mn and Pb (warm and wet season) and Mn and Fe (warm and dry season). Positive correlation of metals indicate common source of metals. As shown in the results, after the rainy season (Warm and Dry Season), there was a decrease in levels of metals across at all the sites with exception of Fe, Mn and Zn. The differences observed after rains for Fe, Mn and Zn could represent a more recent accumulation of these metals after the rainy season.
From the result as presented for the metals (Tables 9-12), it is observed that the concentrations of Cd and Zn in the soil during warm and wet, and warm and dry seasons across the sites is above 1 - 3 and 50 - 300 mg/kg recommended for these metals in soil by European Standards . The values in this study is higher than 0.27 - 1.47 and 32.30 - 98.50 mg/kg reported in soils [9] . A study by [30] on heavy metal content of agricultural soils recorded lower levels of Cd in the soil ranging from 0.150 - 0.880 mg/kg, hence, concentration above EU limits could reflects the influence of human activity. The concentrations of Cr, Cu, Mn, Ni and Pb were within the ranging from 104.30 - 230.00, 58.30 - 207.50, 51.67 - 97.50, 39.30 - 99.50 and 60.00 - 143.30 mg/kg recorded for Cr, Cu, Mn, Ni and Pb, respectively in irrigated soils [9] . Similarly, the values recorded for these metals are below 400, 140, 300, 50, 300 mg/kg the maximum permissible levels for soils recommended by [31] and EU for the metals.
![]()
Table 12. Concentration (mg/kg) of metals in soils during warm and dry season.
na: not available.
![]()
Table 13. Correlation matrix among metals in soils during cool and dry season.
*Correlation is significant at the 0.05 level (2-tailed).
![]()
Table 14. Correlation matrix among metals in soils during hot and dry season.
*Correlation is significant at the 0.05 level (2-tailed).
![]()
Table 15. Correlation matrix among metals in soils during warm and wet season.
*Correlation is significant at the 0.05 level (2-tailed). **Correlation is significant at the 0.01 level (2-tailed).
![]()
Table 16. Correlation matrix among metals in soils during warm and dry season.
*Correlation is significant at the 0.05 level (2-tailed).
The distribution of heavy metals Cd, Cr, Cu, Fe, Mn, Ni, Pb and Zn in the six fractions (water soluble (FI) exchangeable (FII), carbonate bound (FIII), Fe-Mn oxide (FIV), organic bound (FV) and residual (FVI)) for all the samples are summarized in Tables 17-24. The results obtained showed that the amounts of heavy metals extracted from each fraction varied widely among the sites across seasons (p < 0.05).
As observed in Table 17, fractions of Cd in the soil samples among the sites across the seasons showed the range; water soluble: BDL - 0.47 mg/kg with the highest concentration at site 4 during cool and dry season, exchangeable: 0.02 - 0.75 mg/kg with the highest concentration at site 4 during warm and dry season, 0.06 - 0.99, 0.36 - 0.94, 0.17 - 0.98 mg/kg with the highest concentration at site 5 for organic bound, carbonate bound and Fe-MnO fractions during warm and dry season, the residual fraction ranged from 0.42 - 0.92 mg/kg with the highest concentration 0.92 mg/kg found at different sites across the seasons. As observed from the results high-
![]()
Table 17. Chemical fractionation of Cd (mg/kg) in soil samples of irrigation farmlands along Kawo dam.
Value in parenthesis () is the percentage fraction; na: not available.
![]()
![]()
Table 18. Chemical fractionation of Cr (mg/kg) in soil samples of irrigation farmlands along Kawo dam.
Value in parenthesis () is the percentage fraction.
![]()
Table 19. Chemical fractionation of Cu (mg/kg) in soil samples of irrigation farmlands along Kawo dam.
Value in parenthesis () is the percentage fraction.
![]()
Table 20. Chemical fractionation of Fe (mg/kg) in soil samples of irrigation farmlands along Kawo dam.
Value in parenthesis () is the percentage fraction.
![]()
![]()
Table 21. Chemical fractionation of Mn (mg/kg) in soil samples of irrigation farmlands along Kawo dam.
Value in parenthesis () is the percentage fraction.
![]()
Table 22. Chemical fractionation of Ni (mg/kg) in soil samples of irrigation farmlands along Kawo dam.
Value in parenthesis () is the percentage fraction.
![]()
Table 23. Chemical fractionation of Pb (mg/kg) in soil samples of irrigation farmlands along Kawo dam.
Value in parenthesis () is the percentage fraction.
![]()
![]()
Table 24. Chemical fractionation of Zn (mg/kg) in soil samples of irrigation farmlands along Kawo dam.
Value in parenthesis () is the percentage fraction.
est concentration 0.99 mg/kg is associated with the organic bound fraction for Cd in. This situation is however different from [13] , where insignificant amount of Cd are associated with organic bound fraction, whereas in the other fractions Cd concentrations were below the limit of detection. Association of Cd to the organic bound fraction does not generally constitute an environmental risk. This is due to retention of this metal by ion-ex- change proton displacement, and inner and out-spheres complex formation [32] . Consequently, considerable amount of Cd are retained in this fraction, which is less mobile, since it is considered to be associated with higher molecular weight stable and humic substance which release small amounts of metal gradually [33] (1999).
As shown in Table 18, Cr across the sites was dominant in the residual fraction across the seasons (13.68 - 46.72 mg/kg). Highest concentration in the residual of Cr was obtained in site 5 in warm and dry season, then in the Fe-MnO fraction (2.38 - 5.80 mg/kg). Highest concentration of Cr is associated with residual fraction. Small amount of Cr associated with water soluble fraction are noticed in all the sites. This is similar to [34] where no Cr was detected in the first three fractions. According to them, the leaching of Cr to the environment from these samples may not occur readily. Cr (VI) is a highly toxic metal that has been linked to cancer in humans following prolonged inhalation, and is toxic to plants at relatively low concentrations [35] .
For Cu (Table 19), the residual (site 3) and organic bound (site 1) fractions exhibited the highest concentration of 41.33 and 3.83 mg/kg in cool and dry, and warm and wet season, respectively. Dominant level of Cu in the exchangeable phase at site 2 was observed, while in warm and dry season, the carbonate bound fraction dominated at site 5.
Also, the predominant form of Cu available in the entire fractions is residual, exchangeable and carbonate bound fractions. The high % of Cu in the residual fraction is due to stable nature of the compound and the fact that the metals are bonded firmly within a mineral lattice restricted the bioavailability of this metal [36] [37] . This coincides with the researches carried out on soils in China [38] [39] .
Fe across the sites was also associated mostly with the residual phase followed by Fe-MnO and organic bound fractions as shown in Table 20, the concentration ranged 630.33 - 3173.40, 8.35 - 1030.24, 16.01 - 92.98 mg/kg, respectively across the seasons. Highest concentration of 3173.40 mg/kg was recorded in the residual fraction at site 2 in warm and wet season. Though Fe is not toxic heavy metals, it analysis in the present study indicates its predominance of all the metal in all sites. Since the Fe concentration is very high in the residual fraction, the residual fraction could be converted to reducible fraction by the activity of plant roots, hence, available for plant uptake [40] .
The behaviour of Mn was quite different from other metals in that percentage fractions were mainly associated with the exchangeable fraction of the soil samples (site 1) ranging from 0.20 - 13.52 mg/kg (Table 21). Availability of Mn in soils pose no threat to the plants especially at low concentrations recorded in the study.
As shown in Table 22, Ni across the sites was dominant in the residual fraction across the seasons (4.62 - 115.70 mg/kg). Highest concentration in the residual of Cr was obtained in site 5 in cool and dry season, then in the Fe-MnO fraction (2.40 - 6.71 mg/kg).Residual, Fe-MnO and organic bound fraction is the predominant form of Ni in all the samples in the cool and dry season. 1.31 - 7.36 and 0.66 - 7.64 mg/kg is in the exchangeable and carbonate fractions, respectively which can cause environmental toxicity during mobility [41] . Similar to this study, [41] showed that Ni in soils was concentrated in residual fractions. However, in this study, toxicity of Ni is not important because of its low concentration in the mobile and bioavailable fractions.
Highest concentration in residual of Pb was obtained in site 1 during the cool and dry season, then in the Fe-MnO fraction (2.49 - 5.14 mg/kg) as shown in Table 23. Furthermore, with the exception of cool and dry season, lowest level of Pb was observed in the organic bound fraction. From the results, Pb in the soils was found at concentration range of 148.20 - 149.80 mg/kg in residual fraction during the cool and dry season across the sites, while, smaller amount was bound to water soluble and Fe-MnO fraction. A similar distribution of Pb forms among fractions was reported for the fluvial deposits by [42] .
As for Zn, significant amounts of the metal were associated with the residual fraction (Table 24) especially in cool and dry, however, in hot and dry season, highest concentration of Zn was associated with organic bound fraction (100.95 mg/kg). In warm and dry season, the dominant fraction across the sites was Fe-MnO (155.16 - 155.83 mg/kg). Higher concentration of Zn in Fe-MnO fraction can be attributed to diffusion mechanism [43] . This metal can be release into the environment under extremely reducible conditions [34] .
4. Conclusions
1) The physicochemical parameters of the soils across the sites indicated values reported for less polluted soils.
2) With the exception of Cd, all metals evaluated in this study were lower than recommended standard limits.
3) The results of sequential extraction of heavy metal in soil sample indicated that all metals were mainly associated with the residual, Fe-MnO and organic bound fractions. The residual fraction had the maximum concentration of metals especially in cool and dry season for Cr, Cu, Ni, Pb and Zn, whereas only a small fraction of all the heavy metal was extracted in water soluble, exchangeable and carbonate bound fractions. It indicated that the bioavailability index was low. Hence, mobility of the heavy metals by the surrounding plants grown on the soils was low.
Acknowledgements
The authors wish to acknowledge the effort of Mr. Ilu of Soil Chemistry Laboratory, Ahmadu Bello University, Zaria in the soil sample analyses.
NOTES
![]()
*Corresponding author.