Optical Spectroscopic Investigation of TiO2-Like Polymerization Processes by Inductively Coupled Plasma ()


1. Introduction
In the inductively coupled plasma, the electric power is coupled to the electrons via the electromagnetic field; the electron impacts and the molecule/atom collisions are responsible for all kinds of ions, radicals, photons, excited particles, etc. through different reactions of excitation, ionization, and light production. With optical emission spectroscopy, it is possible to better understand the physical properties of the plasma by studying the variation of spectral lines and molecular bands under the different experimental conditions [1]-[3]. So optical emission spectrometry is a unique non-invasive tool to analyze the chemical composition as well as the physical properties of the plasma discharge [4].
In this paper, a system study of the evolution of the emitting plasma species in argon-titanium isopropoxide mixture during the processes of inductively coupled plasma polymerization is analyzed for the different flux of argon gas. Results from the profiles of the high resolution emission spectral for several species, such as H, Ar, and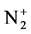 , will be presented and compared in order to understand the plasma-polymerization process.
, will be presented and compared in order to understand the plasma-polymerization process.
2. Experiments
The plasma reactor is showed in Figure 1. A planar plasma source (ICP-P 200, JE Plasma Consult GmbH,
![]()
Figure 1. Plasama source with injection system
German) is equipped with a RF generator (Cescar 1310, Dressler HF technik GmbH). This system can operate with a frequency of 13.56 MHz in the 0.25 - 100 Pa pressure range for the efficient production of a high density, low temperature plasma.
The sample holder is mounted on an electrically insulated manipulator connected to an electrical feed through so that a bias voltage can be applied to the sample holder if necessary. The distance between the sample holder and the quartz window of the plasma source is fixed at 12 cm. The reactive gas is injected through a 20-cm-di- ameter in-house made shower ring. The ring is located inside the vessel and close to the plasma source. This chamber is equipped with a view-port that is transparent for the visible light.
The deposition was carried out on silicon substrate at a RF input power of 400 W, duty cycle 50% (Ton = 0.5 ms, Toff = 0.5 ms), with a different flux of gas. The flacon containing titanium isopropoxidemonomer (Titanium (IV) isopropoxide, Sigma-Aldrich) is heated in 60˚C. Argon gas as carrier brings monomer gas/vapor into the chamber by a transport tube terminated with a “shower ring” output, allowing a homogeneous gas distribution in the discharge region of the chamber. The flow of monomer was controlled by varying the microflow controller and the gas flux was adjusted and measured by Multi gas controller 647 c, MKS. The actual pressure in the chamber can be measured by pressure meter (MKS-Baratron). Before the plasma was carried out, the chamber was cleaned and pumped until 10−3 mbar.
The light emitted by the plasma is coupled by the optical fibre bundle (25-degree solid angle, 19 brins of 200 µm) to the entrance slit of the spectrograph. The plasma gas located between the shower ring and the substrate can be investigated. The resolution can get to 0.05 nm with 1200 grooves/mm grating so that this apparatus allows us to study emission lines in the 200 nm to 1100 nm range with a spectral resolution sufficient for acquisition of ro-vibrationnal molecular band. More detail can be found in [5] and [6].
3. Results and Analysis
The spectra observed for the mixture of argon and titanium isotropropoxide was showed in Figure 2. The atom-
![]()
![]()
Figure 2. Survey of spectrum in the range of 300 nm - 800 nm at 400 W with Ar flux at 10 sccm
ic emission spectra are clearly dominated by neutral Ar atomic lines between 500 - 850 nm (such as 772.4 nm, 794.8 nm, 800.7 nm, and 801.4 nm) that correspond to (4p → 4s) transitions (the so-called “red” lines).
The atomic lines of titanium were observed with very weak intensity, and it is difficult to note them in our figure. It is noted that the impurities are often detected. Many nitrogen molecular bands are observed in the range of 300 nm - 500 nm with the different vibration translation. CO bands can be observed between 400 nm and 600 nm. The intensity of atomic lines and molecular bands varies with the parameters of plasma.
In Figure 3, the variation of intensity as function of Ar flux was observed for species in plasma: Ar, H and CO. All the intensities of the species increase with the Ar flux. But, the intensity of Ar lines increases rapidly compared with these of H atomic line and CO molecular band.
The calculation and treatment of the spectra is based on methods that employ a partly resolved electronic structure of atomic lines, as well as unresolved rotationally and partly resolved rotational electronic structures of bands of molecular spectra. The spectrum of the bands of the second positive system N2 (C3Πu-B3Πg) and the band of the molecular nitrogen ion 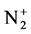 (Δv = 0 sequence of the first negative
(Δv = 0 sequence of the first negative 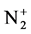 (B2Π+u(v’ = 0)-X2Π+g(v” = 0)) system) are used as diagnostic tool to extract the kinetic temperature from the progression of the rotational and vibrational transitions.
(B2Π+u(v’ = 0)-X2Π+g(v” = 0)) system) are used as diagnostic tool to extract the kinetic temperature from the progression of the rotational and vibrational transitions.
With the best temperature fitted (Trot = 700 K), Figure 4 illustrates comparison the experimental and synthetic spectra of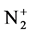 . This comparison was performed under the assumption of Boltzmann distribution over rotational levels. It is assumed that the rotational quantum number is conserved during the electron impact excitation process, and the rotational levels in ground states are populated by heavy particle collisions, rotational temperature can be considered as gas temperature ([7] and [8]). The
. This comparison was performed under the assumption of Boltzmann distribution over rotational levels. It is assumed that the rotational quantum number is conserved during the electron impact excitation process, and the rotational levels in ground states are populated by heavy particle collisions, rotational temperature can be considered as gas temperature ([7] and [8]). The 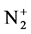 rotational distribution can be considered to obtain gas temperature (Trot = Tg) [9].
rotational distribution can be considered to obtain gas temperature (Trot = Tg) [9].
On the other hand, several effects contribute into the broadening of spectral lines. By Doppler effect, the profile of Hα line is analyzed for gas temperature in plasma. Doppler broadening leads to a Gaussian profile, with FWHM [9] [10].
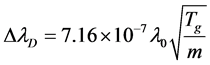
where λ0 = 656.2 nm is the central wavelength of Hα atomic line, m is hydrogen atomic mass. Temperature from calculation of Doppler Effect for Hα atomic line can be considered as gas temperature due to high collision frequency. Stark effect is responsible for the pressure broadening of spectral lines by charged particles. Stark broadening is profile Lorentz. The shape of Ha line was fitted with Voigt function by using the ΔλG and ΔλL under the consideration of Doppler and Stark effects. This line gave ΔλG = 0.01151 nm for Gaussian component and ΔλL = 0.052 nm for Lorentz component. Doppler broadening gives a gas temperature about 898 K which is higher than that calculated from the molecular band N2 (Tg = 700 K). In our experiments, the profile of Hα line does not vary with the flux of Ar. This means that the gas temperature and the density and the temperature of charged particles under our experimental condition change so litter that they can’t influence the spectral profile.
![]()
Figure 3. Normalization intensity for several Ar atomic lines, Hα line et CO band at 519.7 nm.
![]()
Figure 4. Experimental and synthetic spectrum of 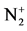 (B2Π+u-X2Π+g) (v’ = 0 → v” = 0) at 391.4 nm.
(B2Π+u-X2Π+g) (v’ = 0 → v” = 0) at 391.4 nm.
4. Conclusion
OES was used to measure the gas temperature in the plasma polymerization process of TiO2-like thin film. The
gas temperature reduced from Hα and N2 band. The profile of spectra lines does not change as a function of the flux of Ar gas. The difference of gas temperature obtained from Hα and N2 band must be reconsidered. The other parameters of plasma will be studied by the analysis of Stark effect for the different atomic lines.
Acknowledgements
This work has been partially supported by China Scholarship council and Natural Scientific Fund of Province Guizhou. Dr Zhiling Li thanks Interdisciplinary laboratory of electronic spectroscopy in University of Namur (FUNDP) for hospitality and Dr N. Bonifaci for the discussion.