Synthesis and Characterization of Poly (Methyl Methacrylate)/Polyethylenimine Grafting Core-Shell Nanoparticles for CO2 Adsorption Using Soap-Free Emulsion Copolymerization ()
Received 14 June 2016; accepted 17 July 2016; published 20 July 2016

1. Introduction
Research on storage and capture of gaseous carbon dioxide (CO2), which is a known cause of global warming, has been going on for decades [1] [2] . Carbon capture and storage refer to techniques for prevention of emissions to reach the atmosphere by adsorption or absorption of the CO2 generated from combustion into materials [3] - [5] . To separate CO2 from the combustibles such as coal or fuel, materials that have the ability to selectively adsorb CO2 are required. CO2 adsorption takes place in the amine functional groups, forming carbonates at a low temperature [6] . Adsorption materials are classified as physical adsorption material, such as zeolite and activated carbon, and chemical adsorption material, such as those prepared by impregnating amine groups of high molecular weight [7] - [12] . Due to high selectivity, large surface area, and monodisperse pores for CO2 adsorption, when the amine with high molecular weight was impregnated into the material, CO2 adsorption capacity becomes high [13] - [15] . However, in case of porous silica used as a carrier for gases, it is difficult to remove the surfactants, and mass production is limited. In this study, to solve the problem, we synthesized core-shell nanoparticles by soap-free emulsion polymerization that uses chemical reactions to graft the methyl methacrylate (MMA) monomers on polyethylenimine (PEI) chains.
Core-shell nanoparticles, which have both hydrophilic and hydrophobic properties, can be prepared by diverse methods and mechanisms allowing simplified incorporation of many functional constituents. Depending on their characteristics, these nanoparticles can be applied to a variety of areas, such as chemical sensors [16] [17] , coatings [18] , and catalysts [19] , in addition to a number of biological applications including biological separation, drug/DNA delivery, gene therapy, enzyme immobilization, and catalysis. In this context, for in vivo use of core-shell particles, it is necessary for the particles to have superior water solubility, biodegradability, bioadaptability, stability, and target specificity. For example, materials with high water solubility that have been used include chitosan, gelatin, poly-D,L-lactide-co-glycolide (PLGA), polyglycolic acid (PGA), polylactic acid (PLA), poly-caprolactone (PCL), and poly-alkyl-cyano-acrylates (PAC). Recently, research has been conducted on PEI, a highly water-soluble cationic polymer that exists in various forms and molecular weights.
Li et al. studied the mechanisms for preparing macromolecules with core-shell structures using MMA and amine-containing water-soluble materials, such as casein, PEI, chitosan, gelatin, poly(allylamine), polyvinylpyrolidone, and poly(vinylamine) [20] [21] . These particles were experimented for gene delivery and intracellular transmission. Pimpha et al. and Inphonlek et al. prepared core-shell macromolecules with gene carrier and antibacterial properties by reacting acid treated chitosan or a solution of PEI-chitosan with PMMA [22] .
The physical and chemical properties of PEI, and its large number of amine functional groups enable adsorption of heavy metals by chelation. PEI can also be used for adsorption of gases, such as CO2, N2, and sulfur. Wu et al. studied adsorption of heavy metals using PMMA/PEI core-shell particles [23] . Ghoil et al. prepared two types of silica/PEI: silica coated with PEI, and silica coated with glutaraldehyde-crosslinked PEI, and compared their performance as heavy metal adsorbents [24] .
In studies on CO2 adsorption by PEI, a porous, inorganic material is usually impregnated with PEI [25] - [29] . However, owing to the characteristics of PEI, elution of PEI can occur at high temperatures or during long-term service. Therefore, research to link impregnated PEI to porous materials more effectively is important.
In this sense, PEI is an interesting material that can be used in diverse areas, and so, frequent studies using this material in biology, adsorbents, catalysis, coatings, adhesive materials, and sensors are being carried out. Most of these studies investigated the performance and efficiency of a particular molecular weight of PEI and for a specific application. However, the effects of changing the molecular weight of PEI on the formation of core-shell particles have not been previously reported. In this study, we induced polymerization of PEI via its amine functional groups to obtain spherical particles with a PEI shell surrounding a macromolecular particle core. We investigated the shapes and sizes of the core-shell particles by varying the molecular weight of PEI and the composition of the emulsion denoted by the PEI/MMA weight ratio.
The motivations for preparing the grafted PMMA/PEI core-shell nanoparticles by soap free emulsion polymerization are as follows. First, to synthesize PMMA/PEI grafting core-shell nanoparticles we introduce the method of soap-free emulsion polymerization, which makes removal of surfactants easy. Second, the presence of PEI on the shell surface provides a technique for controlling the CO2 adsorption sites (that is, the amine functional groups).
2. Materials and Methods
2.1. Materials
Methyl methacrylate (MMA, Junsei) was pre-treated using an inhibitor remover (Sigma Aldrich) to remove polymerization inhibitors. Branched PEI (Mn = 600, 1800, and 10,000) and tert-butyl hydroperoxide (TBHP, 70 wt% in water) were obtained from Sigma Aldrich and used without further purification. Hydrochloric acid (HCl, Sigma Aldrich) was diluted with tertiary distilled water to a concentration of 2 M and used for adjusting the pH of PEI.
2.2. Synthesis of PMMA/PEI Grafting Core-Shell Nanoparticles
PMMA/PEI core-shell nanoparticles were prepared in double-jacketed glass reactor equipped with a mechanical stirrer and tree inlets. First, PEI 10K (0.84 g, Mn = 10,000) was dissolved in distilled water and the solution was adjusted to a pH of 7 using 2 M HCl. MMA monomers (3.36 g) were added to the PEI 10K solution. Then, this solution was purged with N2 gas to remove water and oxygen. After adding the initiator, TBHP (0.25 mL, 10 mM), to the solution, the reaction mixture underwent polymerization by heating for 6 h in an oil bath at 80˚C under a nitrogen atmosphere. The conditions for the synthesis of soap-free PMMA/PEI core-shell nanoparticles such as MMA/PEI weight ratios and PEI molecular weights are presented in Table 1. The nanoparticles prepared using PEI 0.6K (Mn = 600) and PEI 1.8K (Mn = 1800) were polymerized using the same procedure with emulsion composition, MMA/PEI (w/w) ratios, of 1:1 or 4:1. After completion of polymerization, the unreacted starting materials were removed by centrifugation in methanol. The product was then dried at 30˚C in a vacuum oven.
2.3. Characterization
To confirm grafting of PEI and PMMA, we analyzed the core-shell particles using a Fourier-transform infrared spectrometer (FT-IR, MAGNA 550 series II, Nicolet) with a KBr disk. Scanning electron microscopy (SEM, JSM-6701F, JEOL) was used to investigate the surface relief, size, and shape of the core-shell particles.
The SEM samples were prepared by collecting particles directly on carbon tape, or by dissolving the particles in solvent, followed by pipetting 2 - 3 drops on a glass slide, drying, and coating with platinum.
The core-shell structure of the nanoparticles was analyzed using a transmission electron microscope (TEM, JEM-2100F, JEOL). The TEM samples were prepared by dissolving the particles in a solvent, and 1 - 2 drops were transferred onto a carbon-coated copper grid before drying.
A 2% solution of phosphotungstic acid (PTA) was used to stain the samples before analysis. The size and behavior of the prepared nanoparticles in solution were analyzed using a particle size analyzer (PSA, ELS-Z, Otsuka) with tertiary distilled water or methanol as the solvent.
CO2 adsorption capacities of PMMA/PEI grafting core-shell nanoparticles was measured by thermogravimetric analysis (TGA) with the difference of weight under a CO2 atmosphere at 75˚C for 2 hours.
3. Results and Discussion
3.1. Mechanism of Synthesis of PMMA/PEI Grafting Core-Shell Nanoparticles
Figure 1 shows a schematic flowchart of reactions that produces grafting of PMMA/PEI for the synthesis of core- shell nanoparticles. The reactions between the redox initiator, TBHP, and PEI produce free radicals, which induce
![]()
Figure 1. Schematic representation of the formation of PMMA/PEI grafting core-shell nanoparticle.
![]()
Table 1. Conditions for synthesis of PMMA/PEI grafting core-shell nanoparticles.
polymerization of the MMA monomers. Micelle-type nuclei are formed through the grafting reactions between PEI and MMA, and these nuclei grow spontaneously through homopolymerization of MMA and self-assembly, resulting in nanoparticles with a core-shell structure. Soap-free emulsion polymerization has the advantage that it can get cleaner latex than conventional emulsion polymerization. The mechanism for the formation of micelle depends on the oligomer radical with activated surface, which is only slightly soluble in the aqueous medium, to decompose the hydrophilic initiator. The mechanism for the formation of amphiphilic core-shell nanoparticles using a redox initiator has been described in numerous studies. The mechanism is as follows: When an amine group of PEI reacts with TBHP to generate a redox pair, a single electron is transferred from the nitrogen atom of the amine group to TBHP, forming a nitrogen radical cation and a tert-butoxy radical. Following this, the amino radical on PEI is produced as nitrogen loses a single proton, initiating MMA polymerization, while the grafting reaction between PEI and PMMA generates amphiphilic macro-radicals. The tert-butoxy radical can initiate homopolymerization of MMA, while removing a hydrogen atom from the amine group of PEI to generate macro-radicals. These amphiphilic PEI-graft-PMMA macro-radicals self-assemble to form macromolecular micelles consisting of hydrophobic MMA surrounded by hydrophilic PEI. This facilitates homopolymerization of MMA in the interior, which results in the nanoparticle with a PMMA/PEI core-shell structure.
Figure 2 shows FT-IR spectra of PMMA/PEI core-shell nanoparticles prepared in emulsions of various MMA/ PEI ratios. As shown in Figure 2, the carbonyl (C=O) stretching and ether (-OCH3) peaks were observed at 1731 and 1150 cm−1, respectively, corresponding to the molecular structure of PMMA. In addition, the peaks at 3440 (N-H stretching), 2950 (CH2 asymmetric stretching), 2842 (CH2 symmetric stretching), and 1629 cm−1 (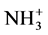 vibration) correspond to PEI, demonstrating successful grafting of MMA and PEI.
vibration) correspond to PEI, demonstrating successful grafting of MMA and PEI.
3.2. Morphology of PMMA/PEI Grafting Core-Shell Nanoparticles with Various MMA/PEI Contents and PEI Molecular Weights
Table 2 shows a comparison of the PMMA/PEI nanoparticle size measured by PSA (in aqueous medium and methanol) and SEM analysis. As shown in Table 2, the average size of the PMMA/PEI nanoparticles measured using PSA is larger than those measured using SEM by about 30 - 60 nm. The increase in particle size can be ascribed to solvent induced swelling of PEI in the shell, which has a good solubility in both water and methanol, resulting in bigger particles than that expected in a dry environment. In addition, PMMA is known to swell in low molecular weight alcohols, such as methanol, ethanol, n-propanol, iso-propanol, and iso-butane. Thus, we conclude that the size of nanoparticles varies depending on the nature of the solvent and the method of measurement.
Figure 3 and Figure 4 show the SEM and TEM images of PMMA/PEI grafting core-shell nanoparticles prepared with different ratios of MMA and PEI 10K. As shown in Figures 3(a)-(c), the weight ratios of MMA/PEI are 1, 4, and 6, respectively. The average size of core-shell nanoparticles gradually increases in accordance with
![]()
Figure 2. FT-IR spectra of PMMA/PEI grafting core-shell nanoparticles prepared by various MMA/PEI ratio.
![]() (a) (b) (c)
(a) (b) (c)
Figure 3. SEM images of PMMA/PEI grafting core-shell nanoparticles prepared with various MMA/PEI ratios: (a) MP10K-11; (b) MP10K-41; and (c) MP10K-61.
![]() (a) (b) (c)
(a) (b) (c)
Figure 4. TEM images of PMMA/PEI core-shell nanoparticles prepared with various MMA/PEI ratios: (a) MP10K-11; (b) MP10K-41; and (c) MP10K-61.
![]()
Table 2. A comparison of PMMA/PEI nanoparticle size measured by PSA and SEM analysis.
MMA/PEI ratios. As shown in Figure 4, the nanoparticles prepared with various MMA/PEI ratios can be confirmed with core-shell structure. The average size of the PMMA/PEI core-shell nanoparticles is shown in Table 2. In the soap-free emulsion polymerization, the size of the nanoparticles is determined by the stability of solution and growth rate of nuclei. In other words, as the ratio of MMA/PEI gradually increased, the amount of PEI relatively decreased. Therefore, the number of nuclei produced in the early stages became lower.
To investigate the effect of PEI molecular weight on PMMA/PEI core-shell nanoparticles, we synthesized the nanoparticles by varying the PEI molecular weights, as shown in Table 3. The amounts of TBHP, PEI, and water used were identical, regardless of the MMA/PEI ratio. As shown in Figure 5, due to the effect of steric hindrance between the high molecular weight PEI and PMMA, a decrease in the size of PMMA/PEI core-shell nanoparticles is observed with an increase in molecular weight of PEI. Likewise, as shown in Figure 6, the nanoparticles prepared with various PEI molecular weights can be confirmed with core-shell structure. Low molecular weight PEI represents shorter molecules than high molecular weight PEI. So, given the same MMA/PEI ratio, low molecular weight PEI molecules in the shell are positioned farther away from PEI molecules in the nearby nanoparticles, and so the aggregation between PEI molecules decreases.
Stability of micelles can be affected by the composition of hydrophilic and hydrophobic regions of the macromolecule, cohesion of the hydrophobic core, and interactions between hydrophilic macromolecular chains in the aqueous solution. In the case of high molecular weight PEI in aqueous solution, the van der Waals forces between PEI molecules, hydrogen bonding with water, and electrostatic interactions can cause the chains to extend, which increases the PEI surface density and sequestering the hydrophobic core to form stable micelles. On the other hand, low molecular weight PEI has relatively low surface density that is insufficient to cover the surface of the micelle and so, as the core is exposed to the aqueous solution, the micelle becomes unstable.
As mentioned above, the use of lower molecular weight PEI results in unstable micelles, generating inhomogeneous nanoparticles. Therefore, we expect that for a low molecular weight PEI, a higher amount of PEI would be required to obtain more stable micelles and homogeneous nanoparticles.
3.3. CO2 Adsorption Capacity of PMMA/PEI Grafting Core-Shell Nanoparticles
Figure 7 shows the CO2 adsorption capacity of PMMA/PEI grafting core-shell nanoparticles prepared using PEI 10k by soap-free emulsion polymerization. The values of CO2 adsorption capacity are presented in Table 4. Particles prepared by soap-free emulsion polymerization exhibit poor CO2 adsorption capacity. We can confirm this value to be less than 0.07 mg per g of absorbent. In Figure 7, we compared the CO2 adsorption capacity of PMMA/PEI grafting core-shell nanoparticles prepared using PEI 10k in accordance to time. The CO2 adsorption capacities of the MP10k-11 and MP1.8k-41 samples were approximately 0.4 mg per g of adsorbent. This can be described as a diminutive CO2 adsorption performance. As mentioned above, PMMA/PEI grafting core-shell nanoparticles have a low CO2 adsorption capacity. The hydrochloric acid, which is used in the preparation of soap-free emulsion at pH = 7, converted all the -NH2 bonds required for CO2 adsorption to quaternary ammonium salts. Therefore, the CO2 adsorption capacity was found to be relatively low.
We, further, synthesized PMMA/PEI grafting core-shell nanoparticles with -NH2 functional groups in emulsions of pH = 11. As a result, CO2 adsorption capacity of the nanoparticles increased to 0.69 mg. Though the CO2 adsorption capacity of the PMMA/PEI core-shell nanoparticles is quite low, it could possible get be further improved if the quaternary ammonium salts in the PEI shell surface was eliminated by pH control using hydrochloric acid.
![]() (a) (b) (c)
(a) (b) (c)
Figure 5. SEM images of PMMA/PEI core-shell nanoparticles prepared with various PEI molecular weights: (a) MP0.6K-41; (b) MP1.8K-41; and (c) MP10K-41.
![]() (a) (b) (c)
(a) (b) (c)
Figure 6. TEM images of PMMA/PEI core-shell nanoparticles prepared with various PEI molecular weights: (a) MP0.6K-41; (b) MP1.8K-41; and (c) MP10K-41.
![]()
Figure 7. CO2 adsorption capacity of PMMA/PEI grafting core-shell nanoparticle prepared using PEI 10k in according to the time.
![]()
Table 3. Conditions for synthesis of PMMA/PEI grafting core-shell nanoparticles using PEI of different molecular weights.
![]()
Table 4. A comparison of CO2 adsorption capacities of PMMA/PEI grafting core-shell nanoparticles.
4. Conclusions
We synthesized PMMA/PEI grafting core-shell nanoparticles using soap-free emulsion polymerization method from hydrophilic PEI and hydrophobic PMMA using radicals from the reaction between the redox initiator TBHP and PEI. As we increased the MMA/PEI weight ratio in the emulsion, the size of the PMMA/PEI grafting core- shell nanoparticles became larger. This result was explained by the lower amount of radical initiator relative to monomers at the initiation stage of the reaction. When we varied the molecular weight of PEI, the size of the nanoparticles decreased as PEI molecular weight increased, and the distribution of particle size was found to be more homogeneous for high molecular weight PEI. Moreover, regardless of the molecular weight of PEI, when the amount of PEI was increased, nanoparticle aggregation was observed, and the size of PMMA/PEI grafting core-shell nanoparticles was gradually increased by the amount of MMA added in the emulsion. From the results of this study, it will be possible to prepare environmentally-friendly nanoparticles without surfactants that are normally required. In the future, further applications of core-shell nanoparticles can be expected as the size and properties of the particles can be better controlled using molecular weight of PEI and PEI/MMA ratio.
Finally, a maximum CO2 adsorption capacity of 0.69 mg per g of adsorbent was found for the PMMA/PEI grafting core-shell nanoparticles. Though this CO2 adsorption capacity does not have the higher CO2 adsorption capacity, in another aspect, we confirmed the possibility of a follow-up study.
Acknowledgements
This study has been conducted with the support of the Korea Institute of Industrial Technology as “Platform Technology for Preparation and Process of High Efficient and Functional Materials (kitech JA-16-0360)”. This research was additionally supported by Technology Commercialization Support Program, Ministry of Agriculture, Food, and Rural Affairs.