Augmentation of Dry Matter Production, Photosynthetic Enzymes, Yield Attributes and Quality Parameters of Sunflower through Seed Priming Effect of Gibberellic Acid—A Multifaceted Hormone ()
Subject Areas: Metabolic Sciences, Plant Science

1. Introduction
Sunflower (Helianthus annus L.) is one of the annual oil crops of India. It is highly desirable for its premium oil, to supplement our oil seeds production, and contributes about 24% of the domestic edible oil production. The oil contains high degree of polyunsaturated fatty acids and anticholestric properties [1] . The efforts have been made today to increase its productivity by adopting the scientific agro-practices and by overcoming the incomplete development of seeds. Majority of farmers in our country has marginal holdings of less than two hectares, a major problem to boost-up its productivity. Keeping in mind such a limitation on increasing its productivity, it is highly desirable to find out ways which can augment the yield of this valuable oil yielding crop.
To meet the challenges of the low production and local requirements, there is need for multipronged strategy. In this context, efforts in the form of launching national programmes and research projects have been made by governmental and non-governmental organizations. The considerable number of fertilizers is rendered unavailable to the plants due to many factors, including leaching, fixation, decomposition and volatilization. For example, up to 50% of the soil-applied N [2] , about 70% of the soil-applied P [3] and more or less 60% of the total applied sulphur (S) [4] - [6] may be lost due to one or more of these reasons. As mentioned earlier, there is limitation on increasing the acreage for cultivation, it is, therefore, highly logical to innovate ways that can improve the sunflower productivity. In this regard, an approach could be to make plants utilize fully the available resources leading to maximum harvesting of solar energy and subsequently enhancing the active sites. To attain such goal, the use of plant growth regulators (PGRs) may play an important role as they are known to affect many facets of plant life, including photosynthetic rate , N-fixation, water and mineral uptake, harvest index (HI) [7] . Gibberellins are a class of endogenous plant growth substances exert pleiotropic effects on developmental processes like leaf expansion, stem elongation, cell collation and cell differentiation by co-ordinating with other PGRs like auxin, cytokinin, salicylic acid and triacontanol etc., [8] - [11] . Moreover, application of GA improves, among other processes, absorption and use efficiency of nutrients [12] [13] , activity of enzymes [14] [15] , cell division, cell enlargement [16] [17] , chlorophyll content [15] , elongation of internode [18] , membrane permeability [17] [19] , PN [20] , nucleic acid and protein synthesis [18] [21] , and transport of photosynthates [22] - [24] .
, N-fixation, water and mineral uptake, harvest index (HI) [7] . Gibberellins are a class of endogenous plant growth substances exert pleiotropic effects on developmental processes like leaf expansion, stem elongation, cell collation and cell differentiation by co-ordinating with other PGRs like auxin, cytokinin, salicylic acid and triacontanol etc., [8] - [11] . Moreover, application of GA improves, among other processes, absorption and use efficiency of nutrients [12] [13] , activity of enzymes [14] [15] , cell division, cell enlargement [16] [17] , chlorophyll content [15] , elongation of internode [18] , membrane permeability [17] [19] , PN [20] , nucleic acid and protein synthesis [18] [21] , and transport of photosynthates [22] - [24] .
Keeping in mind, the importance of this valuable oil producing crop, a pot experiment was conducted to investigate the effects of seed soaking (seed priming) effects of GA on growth characters, photosynthesis, enzyme activities, nutrient contents and yield attributes as well as quality parameters of sunflower. Therefore, this present work done to study, keeping in mind the immerse prospect of the crop like to increase its productivity especially in relation to seed yield and oil quality. Thus, the major goals of this research work are to determine the well-known effectiveness of GA treatments in enhancing productivity and yield of sunflower in India.
2. Materials and Methods
2.1. Soil Analysis
Just before sowing a composite soil sample, collecting from each pot, was analyzed for the soil characteristics. The soil sample was analyzed in the soil Testing Laboratory, Government Agriculture Farm, Quarsi, Aligarh. The physico-chemical properties of soil are given Table 1.
2.2. Plant Materials, Growth Conditions and Experimental Design
A pot experiment was conducted on sunflower (Helianthus annus L.) during the zaid (summer) season in a net house of the department of Botany, Aligarh Muslim University, Aligarh. It is situated at 27˚52'N latitude, 78˚. The aim of this experiment was to study the effect of four concentrations and four soaking durations of pre-
![]()
Table 1. Physico-chemical characteristics of the soil mixture used for the experiment.
sowing seed treatment of GA on the performance of sunflower cv. PAC 3776. The sunflower variety cv. PAC 3776 is characterized by one of the fastest growing oil seed crop in India, more economic in nature, also used as a ornamental plant, low irrigation requirement, minimum fertilizer and manures need but give well responses, and adopted to most of the soil category of India. The seeds obtained from the Advanta India, Sikandarabad (A.P), were surface-sterilized by soaking in 0.01% HgCl2 solution for 3 min, washed thoroughly with distilled water and divided into sixteen sets. Seeds are soaked in distilled water containing 10−7, 10−5 and 10−3 M GA respectively, for 4 h, 8 h, 12 h and 16 h. After soaking, the thoroughly washed seeds were planted (10 per pot) in earthen pots (25 cm diameter) filled with 4 kg homogenous mixture of soil and farmyard manure (FYM) in the ratio of 4:1. The pots were arranged in a factorial randomized design. Each pot was supplied with NPK at the rate of 40 kg N + 60 kg P + 40 kg K/ha at the time of sowing. A half dose of N was applied at 25 DAS and the remaining half dose was given at 50 DAS. After germination only four uniform seedlings were left in each pot. Each treatment was replicated four times. The performance of the crop was assessed with regard to dry weight of shoot and root per plant, LAI per plant, CA activity and NR activity NPK content, seeds per head, seed yield per plant, HI, acid value, iodine value and saponification value of oil at harvest.
2.3. Determination of Growth Characteristics
The shoot and root of each plant were dried in a hot air oven at 80˚C for 24 h and their dry weight was obtained separately with the help of an electronic balance. LAI is the ratio of foliage area to ground area. It is determined by the following formula suggested by Watson [25] .
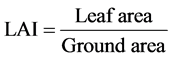
2.4. Determination of Physiological and Biochemical Parameters
The activity of CA (E.C.4.2.1.1) determined in fresh leaves collected randomly from each replicate. The enzyme carbonic anhydrase is responsible for the catalysis for the reversible hydration of carbon dioxide (CO2) to give the bicarbonate (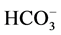 ). The activity of the enzyme was estimated by adopting the method [26] . The collected leaves were cut into the small pieces (1 cm2) at a temperature below 20˚C. After mixing them, 200 mg leaf pieces were weighed and cut further into smaller pieces keeping them in 100 ml 0.2 M aqueous cysteine hydrochloride solution in a Petri-dish at 0˚C to 4˚C for 20 min. The solution adhering on their surface was thin removed with the help of a blotting paper. This was followed by transfer immediately into a test tube having 4 ml phosphate buffer of PH 6.8. To this, 4 ml 0.2 M sodium bicarbonate (in 0.2 M sodium hydra-oxide solution) and 0.2 ml of 0.002% bromothymol blue indicator were added. After shaking, the tubes were kept at 0˚C - 4˚C for 20 min. CO2 liberated during catalytic action of the enzyme on sodium bicarbonate was estimated by titrating the reaction mixture against 0.05 N hydrochloric acid, using methyl red as an indicator. A control reaction mixture was also titrated against 0.05 N hydrochloric acid. The difference of the sample reading and blank reading was noted for further calculations of enzyme activity. The activity of the enzyme was calculated by the following formula:
). The activity of the enzyme was estimated by adopting the method [26] . The collected leaves were cut into the small pieces (1 cm2) at a temperature below 20˚C. After mixing them, 200 mg leaf pieces were weighed and cut further into smaller pieces keeping them in 100 ml 0.2 M aqueous cysteine hydrochloride solution in a Petri-dish at 0˚C to 4˚C for 20 min. The solution adhering on their surface was thin removed with the help of a blotting paper. This was followed by transfer immediately into a test tube having 4 ml phosphate buffer of PH 6.8. To this, 4 ml 0.2 M sodium bicarbonate (in 0.2 M sodium hydra-oxide solution) and 0.2 ml of 0.002% bromothymol blue indicator were added. After shaking, the tubes were kept at 0˚C - 4˚C for 20 min. CO2 liberated during catalytic action of the enzyme on sodium bicarbonate was estimated by titrating the reaction mixture against 0.05 N hydrochloric acid, using methyl red as an indicator. A control reaction mixture was also titrated against 0.05 N hydrochloric acid. The difference of the sample reading and blank reading was noted for further calculations of enzyme activity. The activity of the enzyme was calculated by the following formula:
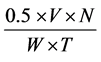 mmol (CO2)/mg(leaf fresh mass)/min
mmol (CO2)/mg(leaf fresh mass)/min
where,
V = Difference in volume (ml) of HCl used in blank and test sample titration
N = Normality of HCl
W = Fresh weight of tissue in mg
T = Duration of the catalytic action of the enzyme (min)
The enzyme, nitrate reductase (E.C.1.6.6.1-3) catalyses the reduction of nitrate (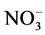 ) to nitrite (
) to nitrite ( ). The nitrate reductase activity in fresh leaves was estimated by the method [27] . The leaves were cut into small pieces (1 cm2). Two hundred mg of these chopped leaves were weighed and transferred into plastic vials. To each vial, 2.5 ml phosphatic buffer of pH 7.5 and 0.5 ml of potassium nitrate solution were added followed by the addition of 2.5 ml of 5% isopropanol. These vials were incubated in a BOD incubator for 2 h at 30˚C ± 2˚C in the dark. 0.4 ml of incubated mixture was taken into test tube to which 0.3 ml each of sulphanilamide solution and NED- HCl were added. The test tube was left for 20 min for maximum colure development. The mixture was diluted to 5 ml by adding DDW. The OD was recorded at 540 nm using the spectrophotometer.
). The nitrate reductase activity in fresh leaves was estimated by the method [27] . The leaves were cut into small pieces (1 cm2). Two hundred mg of these chopped leaves were weighed and transferred into plastic vials. To each vial, 2.5 ml phosphatic buffer of pH 7.5 and 0.5 ml of potassium nitrate solution were added followed by the addition of 2.5 ml of 5% isopropanol. These vials were incubated in a BOD incubator for 2 h at 30˚C ± 2˚C in the dark. 0.4 ml of incubated mixture was taken into test tube to which 0.3 ml each of sulphanilamide solution and NED- HCl were added. The test tube was left for 20 min for maximum colure development. The mixture was diluted to 5 ml by adding DDW. The OD was recorded at 540 nm using the spectrophotometer.
N, P and potassium (K) were estimated in dried powder of leaves obtained from each replicate. The sampled plants leaves were dried in an oven at 80˚C for 24 h. The dried leaves from each sample were finally powdered and then passed through a 72-mesh screen. For the estimation of these nutrients, the leaf powder was first digested according to the standard technique.100 mg oven dried powder of leaf material was transferred into a digestion tube to which 2 ml sulphuric acid was added. The tube was then kept on a digestion assembly at 80˚C for about 2 h to allow the complete reduction of NO3 present in the plant material by the organic matter itself. Initially, dense white fumes were given off and then the content of the tube turned black. After cooling the tube for about 15 min, 0.5 ml 30% hydrogen peroxide (H2O2) was added drop by drop and the tube was heated again till the colour of the solution changed from black to light yellow. The digestion tube cooled for 10 min and an additional amount (2 - 3 drops) of 30% H2O2 was added followed by gentle heating for about 15 min to get a clear and colourless solution. At this stage, care was taken in the addition of H2O2 because its excess might oxidize ammonia in the absence of organic matter. The H2O2 digested material was diluted with DDW and transferred with three washings into a 100 ml volumetric flask and finally the volume was made up to the mark with DDW. The details of methods employed for the estimation of N, P and K are given below.
N was estimated according to the method [28] . A 10 ml H2SO4-H2O2 digested material was taken into a 50 ml volumetric flask and the excess of the acid was neutralized by the addition of 2 ml 2.5N sodium hydroxide. 1 ml 100% sodium silicate was added to prevent turbidity and finally, the volume was made up with DDW. Into a 10 ml graduated test tube, 5 ml this solution was taken and 0.5 ml Nessler’s reagent was added. The content of the test tube were allowed to stand for 5 min for maximum colour developed. The solution was transferred into a calorimetric tube and OD was read at 525 nm, using a blank on the spectrophotometer. N content was determined with the help of the standard curve and was expressed in terms of percentage on dry weight basis.
P content in the H2O2 digested material was estimated [29] . 5 ml H2SO4-H2O2 digested material was taken into a 10 ml graduated test tube and 1 ml molybdic acid was carefully added followed by the addition of 0.4 ml 1-amino-2-naphthol-4-sulphonic acid. The colour of the solution turned blue. The final volume in the tubes was made up to 10 ml with DDW. After mixing thoroughly, the contents of the tube were allowed to stand for 5 min. They were then transferred into a calorimetric tube and OD was read at 620 nm on the spectrophotometer. A blank was run simultaneously for each determination. P content was computed with the help of the standard curve and was expressed in terms of percentage on dry weight basis. K was estimated flame photometrically. 10 ml H2SO4-H2O2 digested material was taken into a vial and was run into a flame photometer (AIMIL “Fotoflame”) using the filter for K. A blank was run side by side. K content was computed with the help of a standard curve and was expressed in terms of percentage on dry weight basis.
2.5. Determination of Yield and Quality Characteristics
The number of seeds per head was determined by counting the number of seeds of four heads. The total seeds of four plants (four heads) were threshed, cleaned and allowed to dry in the sun for some time and their weight was obtained with the help of an electronic balance, with expressing their weight on per plant basis. The HI was computed by dividing the seed yield of a plant by the biological yield of the plant and expressed on per cent basis.
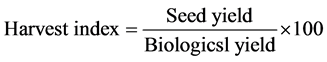
The acid value of oil is the number of mg of potassium hydroxide (KOH) used to neutralize free acids in one gram of oil (mg KOH/g oil). It was determined by the titration method [30] .
2 g oil was dissolved in 50 ml solvent mixture of 95% ethyl alcohol and diethyl ether (1:1) in a 250 ml conical flask. Titration was carried out with 0.1 N KOH using phenolphthalein as an indicator and the amount of ml “a” of 0.1 N KOH required was noted. The acid value was determined by the following formula.
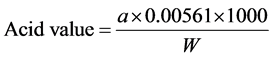
where,
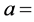 ml of 0.1 N KOH used in titration
ml of 0.1 N KOH used in titration
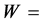 weight of oil in g
weight of oil in g
The iodine value of oil is the number of g of iodine absorbed by 100 g oil (g 1/100g oil). It was determined by using iodine monochloride method describe below [30] .
2 g oil was placed into a dry 250 m1 round bottom flask. 10 ml carbon Iran chloride and 20 ml iodine mono- chloride solution were added. The flask was stoppered and allowed to stand in a dark place for about 30 mm. Thereafter, 15 ml potassium iodide solution and 100 ml DDW were poured into the flask with gentle shaking. Titration was carried out with 0.1 N sodium thiosulphate solutions, using starch solution as an indicator. The number of ml “a” of sodium thiosulphate used was noted. For blank, similar operation was performed but without the oil, and the number of ml “b” of 0.1 N sodium thiosulphate solution used was noted. Iodine value was calculated by the following formula:
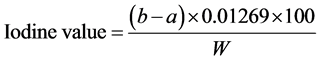
where,
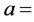 number of ml of 0.1 N sodium thiosuiphate solution used for the sample
number of ml of 0.1 N sodium thiosuiphate solution used for the sample
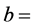 number of ml of 0.1 N sodium thiosulphate solution used for the blank
number of ml of 0.1 N sodium thiosulphate solution used for the blank
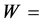 weight of oil in g
weight of oil in g
The saponification value of oil is the number of mg of KOH required to neutralize the fatty acids resulting from the complete hydrolysis of 1 g of oil (mg KOH/g oil). It was determined by titration method [30] . 2 g oil was taken into a 250 ml conical flask to which 25 ml 0.5 N KOH solution was added. The flask was attached with a reflux condenser and heated on a water bath for about 1 h with frequent rotation of the contents of the flask. After cooling, 1 ml phenolphthalein solution was added. The excess of alkali was titrated with 0.5 N HCl and the number of ml “a” was noted. For blank, the operation was repeated in the same manner omitting the oil, and the number of ml “b” required was noted. Saponification value was calculated by following formula:
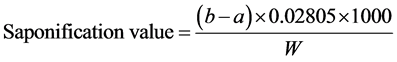
where,
![]() number of ml of 0.5 N HC1 used for the sample
number of ml of 0.5 N HC1 used for the sample
![]() number of ml of 0.5 N HC1 used for the blank
number of ml of 0.5 N HC1 used for the blank
![]() weight of oil in g
weight of oil in g
The data of the experiment were analysed statistically by adopting the analysis of variance technique [31] . For the “F” test, the error due to replicates was also determined. When “F” value was found to be significant at 5% level of probability, critical difference (CD) was calculated.
3. Results and Discussion
3.1. Growth Characteristics
Soaking treatment 10−5 M GA, followed by 10−3 M GA, gave the maximum value at both stages. Soaking with 10−5 M GA gave 125.0% and 52.38% higher value than 0 M GA at 50 and 70 DAS respectively. The effect of soaking durations on shoot dry weight was not found significant at both stages. Interaction 10−5 M GA × 8 h gave the maximum value at both stages. However, its effect was at par with that of 10−5 M GA × 12 h, 10−4 M GA × 16 hand 10−5 M GA × 4 h at both stages and also with that of 10−3 M GA × 8 h at 70 DAS. Interaction 10−5 M GA × 8 h gave 132.98% and 53.99% higher shoot dry weight than the lowest value giving interaction 0 M GA × 4 h at 50 and 70 DAS respectively, Table 2. Soaking treatment 10−5 M GA gave the maximum value for root dry weight at 50 DAS, with its effect being followed by that of 10−3 M GA. Soaking with 10−3 M GA gave 150.41% higher values than 0 M GA at this stage. However, soaking concentrations did not vary in their effect at 70 DAS, Table 3.
![]()
Table 2. Effect of concentrations (C) and soaking durations (h) of pre-sowing seed treatment of GA3 on shoot dry weight per plant (g) of sunflower at 50 and 70 DAS (mean of four replicates).
NS = Non-significant.
![]()
Table 3. Effect of concentrations (C) and soaking durations (h) of pre-sowing seed treatment of GA3 on root dry weight per plant (g) of sunflower at 50 and 70 DAS (mean of four replicates).
NS = Non-significant.
Soaking treatment 10−5 M GA gave the maximum LAI at both 50 and 70 DAS. However, its effect was at par with that of 10−3 M GA and 10−7 M GA at 50 DAS and only with that of 10−3 M GA at 70 DAS. Soaking with 10−5 M GA gave 9.46% and 11.14% higher LAI than 0 M GA at 50 and 70 DAS respectively. Soaking for 8 h proved best at both stages. Its effect was followed at 50 DAS but equalled at 70 DAS by that of 12 h soaking. Soaking for 8 h gave 19.8 1% and 11.35% higher LAI than 4 h soaking at 50 and 70 respectively. Interaction 10−5 M GA × 8 h gave the maximum LAI at both 50 and 70 DAS. However, its effect was at par with that of 10−3 M GA × 8 h, 10−7 M GA × 8 h, and 10−5 M GA × 12 h at 50 DAS and also by 10−5 M GA × 12 h, 10−3 M GA × 12 h and 10−7 M GA × 12 h at 70 DAS. Interaction 10−5 GA × 8 h gave 33.82% and 22.94% higher leaf area index than the lowest value giving interaction 0 M GA × 4 h at 50 and 70 DAS respectively, Table 4.
3.2. Physiological and Bio-Chemical Parameters
Soaking treatment of 10−5 M GA gave the maximum value at both stages for CA activity. Its effect was at par at 50 DAS and followed at 70 DAS by that of 10−3 M GA. Soaking with 10−5 M GA gave 16.07% and 11.07% higher value than 0 M GA at 50 and 70 DAS respectively. Soaking for 8 h proved the best at both stages, however its effect was at par with that of 12 h soaking. Soaking for 8 h gave 21.68% and 33.71% higher value than 4 h soaking at 50 and 70 DAS respectively. Interaction 10−5 M GA × 8 h gave the maximum value for CA activity at 70 DAS, however its effect was at par with that of 10−3 M GA × 8 h. Interaction 10−5 M GA × 8 h gave 44.88% higher value than the lowest value giving interaction 0 M GA × 4 h at 70 DAS. Effect of interactions on this parameter was, however not significant at 50 DAS, Table 5. Soaking treatment of 10−5 M GA gave the maximum value of NR activity at both stages, with its effect being followed by that of 10−3 M GA. Soaking with 10−5 M GA gave 11.68% and 16.70% higher value than 0 M GA at 50 and 70 DAS respectively. Soaking for 8 h proved the best at both stages, with its effect being followed by that of 12 h soaking. Soaking for 8 h gave 11.59% and 12.02% higher value than 4 h soaking at 50 and 70 DAS respectively. Interaction 10−5 M GA × 8 h gave the maximum value at both stages. Its effect was followed by that of 10−3 M GA × 8 h, 10−5 M GA × 12 h, 10−7 M GA × 8 h and 10−5 M GA × 16 h at 50 DAS. However, effect of 10−5 M GA × 8 h was at par with that of 10−3 M GA × 8 h at 70 DAS. Interaction 10−5 M GA × 8 h gave 25.86% and 28.78% higher value than the lowest value giving interaction 0 M GA × 4 h at 50 and 70 DAS respectively, Table 6.
![]()
Table 4. Effect of concentrations (C) and soaking durations (h) of pre-sowing seed treatment of GA3 on leaf area index (LAI) of sunflower at 50 and 70 DAS (mean of four replicates).
![]()
Table 5. Effect of concentrations (C) and soaking durations (h) of pre-sowing seed treatment of GA3 on carbonic anhydrase activity [n mol CO2/kg(leaf FW)/s] of sunflower at 50 and 70 DAS (mean of four replicates).
NS = Non-significant.
![]()
Table 6. Effect of concentrations (C) and soaking durations (h) of pre-sowing seed treatment of GA3 on nitrate reductase activity [n mol ![]() /g/(leaf FW)/h] of sunflower at 50 and 70 DAS (mean of four replicates).
/g/(leaf FW)/h] of sunflower at 50 and 70 DAS (mean of four replicates).
Soaking treatment of 10−3 M GA gave the maximum for leaf N content at both 50 and 70 DAS. Its effect was followed by 10−5 M GA at both stages. Soaking with 10−3 M GA gave 5.67% and 12.80% higher value than 0 M GA at 50 and 70 DAS respectively. Soaking for 8 h proved the best at both stages, however its effect was followed by that of 12 h soaking. Soaking for 8 h gave 13.98% and 10.76% higher value than 4 h soaking at 50 and 70 DAS respectively. Of interactions on this parameter was not found significant at both 50 and 70 DAS, Table 7. Soaking treatment of 10−3 M GA gave the maximum value for leaf P content at 70 DAS. Its effect was followed by that of 10−5 M GA at this stage. Soaking with 10−3 M GA gave 43.30% higher value than 0 M GA at 70 DAS. However, a non-significant effect of soaking treatments was noted at 50 DAS. Soaking for 8 h proved the best at 70 DAS. Its effect was followed by that of 12 h soaking at this stage. Soaking for 8 h gave 24.23% higher value than 4 h soaking at 70 DAS. Duration treatments did not affect on this parameter at 50 DAS. Interaction 10−3 M GA × 8 h gave the maximum value at 70 DAS; however its effect was at par with that of 10−5 M GA × 8 h. Interaction 10−3 M GA × 8 h gave 67.63% higher value than the lowest value giving interaction 0 GA × 4 h at 70 DAS. Interaction effect did not vary at 50 DAS, Table 8.
Soaking treatment of 10−3 M GA gave the maximum for leaf K content at both 50 and 70 DAS. However, its effect was at par with that of 10−5 M GA at both stages and also by 10−7 M GA at 70 DAS. Soaking with 10−3 M GA gave 32.48% and 32.79% higher value than 4 h soaking at 50 and 70 DAS respectively. Soaking for 8 h proved the best at both stages, with its effect being followed by that of 12 h soaking. Soaking for 8 h gave 45.38% and 44.35% higher value than 4 h soaking at 50 and 70 DAS respectively. Interaction 10−3 M GA × 8 h gave the maximum value at both stages. However, its effect was at par with that of 10−5 M GA × 8 h at both stages and also by 10−7 M GA × 8 h at 70 DAS. Interaction 10−3 M GA × 8 h gave 86.14% and 85.71% higher value than the lowest value giving interaction 0 M GA × 4 h at 50 and 70 DAS respectively, Table 9.
3.3. Yield Characteristics
Soaking treatment 10−5 M GA, followed by 10−5 M GA, gave the maximum value of seed per head. Soaking with 10−5 M GA gave 108.51% higher value than 0 M GA. Soaking for 8 h proved the best. Its effect was fol-
![]()
Table 7. Effect of concentrations (C) and soaking durations (D) of pre-sowing seed treatment of GA3 on nitrogen content (%) of sunflower at 50 and 70 DAS (mean of four replicates).
NS = Non-significant.
![]()
Table 8. Effect of concentrations (C) and soaking durations (h) of pre-sowing seed treatment of GA3 on leaf phosphorus content (%) of sunflower at 50 and 70 DAS (mean of four replicates).
![]()
Table 9. Effect of concentrations (C) and soaking durations (D) of pre-sowing seed treatment of GA3 on leaf potassium content (%) of sunflower at 50 and 70 DAS (mean of four replicates).
lowed by that of 12 h soaking. Soaking for 8 h gave 5.32% higher value than 4 h soaking. Interaction 10−5 M GA × 8 h gave the maximum value, however its effect was at par with that of 10−5 M GA × 12 h and 10−5 M GA × 16 h. Interaction 10−5 M GA × 8 h gave 123.49% higher value than the lowest value giving interaction 0 M GA × 4 h, Table 10. Soaking treatment 10−5 M GA gave the maximum value of seed yield per plant. Its effect was followed by that of 10−3 M GA. Soaking with 10−5 M GA gave 42.2% higher value than 0 M GA. The effect of soaking durations on seed yield was not found significant. Interaction of 10−5 M GA × 8 h gave the maximum value, however its effect was at par with that of 10−5 M GA × 12 h, 10−5 M GA × 16 h and 10−5 M GA × 4 h. Interaction 10−5 M GA × 8 h gave 44.76% higher value than the lowest value giving interaction 0 M GA × 4 h, Table 11. Soaking treatment 10−5 M GA gave the maximum value of HI. Its effect was followed by that of 10−3 M GA. Soaking with 10−5 M GA gave 40.28% higher value than 0 M GA. The effect of soaking durations was not found significant on this parameter. Interaction 10−5 M GA × 8 h gave the maximum value, however its effect was at par with that of 10−3 M GA × 12 h, l0−3 M GA × 16 h, 10−3 M GA × 4 h, 10−5 M GA × 8 h, 10−5 M GA × 12 h, 10−5 M GA × 16 h and 10−5 M GA × 4 h. Interaction 10−3 M GA × 8 h gave 41.38% higher value than the lowest value giving interaction 0 M GA × 4 h, Table 12.
![]()
Table 10. Effect of concentrations (C) and soaking durations (D) of pre-sowing seed treatment of GA3 on seeds per head of sunflower at harvest (mean of four replicates).
![]()
Table 11. Effect of concentrations (C) and soaking durations (D) of pre-sowing seed treatment of GA3 on seed yield per plant of sunflower at harvest (mean of four replicates).
![]()
Table 12. Effect of concentrations (C) and soaking durations (D) of pre-sowing seed treatment of GA3 on harvest index (HI) of sunflower at harvest (mean of four replicates).
Soaking treatment 10−5 M GA gave the maximum response for acid value. Its effect was followed by that of 10−3 M GA and 10−7 M GA. Soaking with 10−5 M GA gave 72.88% higher value than 0 M GA. Soaking for 8 h proved best, however its effect was at par with that of 12 h soaking. Soaking for 8 h gave 20.00% higher value than 4 h soaking. Interaction 10−5 M GA × 8 h gave the maximum value. However, its effect was at par with that of 10−5 M GA × 12 h, 10−5 M GA × 16 h, 10−5 M GA × 4 h, 10−3 M GA × 8 h and 10−3 M GA × 12 h. Interaction 10−5 M GA × 8 h gave 111.76% higher value than the lowest value giving interaction 0 M GA × 4 h, Table 13. The effect of soaking concentrations and soaking durations as well as their interactions was not found significant on iodine value, Table 14. The effect of soaking concentrations was not found significant for saponification value. The effect of soaking durations was also not found significant on this parameter. Interaction 10−3 M GA × 8 h gave the maximum value. However, its effect was at par with that of 10−3 M GA × 12 h, 10−3 M GA × 16 h, 10−3 M GA × 4 h, 10−5 M GA × 8 h, 10−5 M GA × 12 h and 10−5 M GA × 16 h. Interaction 10−3 M GA × 8 h gave 1.64% higher value than the lowest value giving interaction 0 M GA × 4 h, Table 15.
![]()
Table 13. Effect of concentrations (C) and soaking durations (D) of pre-sowing seed treatment of GA3 on acid value of the oil of sunflower (mg KOH/g oil) at harvest (mean of four replicates).
![]()
Table 14. Effect of concentrations (C) and soaking durations (D) of pre-sowing seed treatment of GA3 on iodine value of the oil of sunflower (g l/100g) at harvest (mean of four replicates).
![]()
Table 15. Effect of concentrations (C) and soaking durations (D) of pre-sowing seed treatment of GA3 on saponification value of the oil of sunflower at harvest (mean of four replicates).
As is complete evident from the Tables 2-15, plants from the GA treated seeds showed significant enhancement over the control in all parameters studied. The genetic potential of the plants is realized to a great extent by a recognized group of phytohormone or PGR called GA, like all other phytohormones, this also involved or express their potential of regulation of physiological processes of plants by modification of transcription, translation and/or differential sensitivity of the tissues. The receptors for GA are found on the plasma membrane. The difference in performance of sunflower to various concentrations of GA with respect to different soaking periods could be ascribed to the variations caused in their genetic makeup. Maximum stimulation was noted because of 10−5 M GA following 8 h pre-sowing soaking, in which case, the values of shoot and root dry weight and LAI were elevated by 132.98%, 170.59% and 33.82% respectively, over the control at the 50 DAS sampling stage. Similar enhancements of 25.86% and 86.14% for NR and K respectively at the same stage. Moreover, oil quality characteristics like acid value and saponification value were registered an increase of 111.76% and 1.64% respectively over the control, whereas seed yield was elevated by 44.76% following the hormone treatment at harvest.
The observed ameliorative effect of pre-sowing seed treatment with GA particularly at 10−5 M GA over the control on shoot and root dry weight per plant and LAI of 50 and 70 DAS can be traced to its various roles in the plants. The observed increases in growth parameters can be primarily attributed to the optimization of cell wall extensibility. Huttly and Phillips [21] , in their review article, suggested that wall extensibility is the principal responsible factor that control cell expansion which, in turn, may be considered a central detrimental causative agent for growth like activities. In fact, GA has been found to be correlated with increased activity of the enzyme xyloglyconendotrans-glycosylase, which catalyses the breaking and forming of bonds between xyloglycon residues, thus permitting a transient increase in cell wall extensibility, and inducing elongation and cell expansion, which is apparent herein as increased shoot and root dry weight and LAI, Tables 2-4. Increased LAI, in turn, provides increased opportunity for light harvesting, which ultimately manifests as more dry matter. This stance is also supported by the strong positive correlation obtained herein between LAI and dry matter![]() .
.
It is known that CA has an active role in photosynthesis, which is established by its presence in all photosynthesizing tissues, where it catalyzes the reversible hydration of CO2, thereby increasing its availability for Rubisco [32] [33] . GA is already known to have an enhancing effect on the efficiency of Rubisco [13] [34] . Nitrate reducing power of the plant is one of the important factors determining the growth. However, the processes of nitrate reduction is directly, indirectly dependent on the metabolic sensors and/or/signal transducers [35] . The level of enzyme, NR is dependent on a number of factors borned within/outside of the plants. In the present research study, leaves of plants from the GA-treated seeds were found to possess more CA activity than the control, Table 5. A probable cause may be some influence of GA on the de novo synthesis of GA, which involves translation/transcription [36] [37] . GA is in fact known to affect these processes and hence has some control over protein and enzyme synthesis [21] [33] . As such, GA was found to enhance the activity of CA and NR, most probably, by causing an enhancement in its relative concentration in the plant tissues. Enhancement in shoot and root dry weight and LAI were expectedly reflected from increased shoot and root length of treated plants because levels of treated plants possessed a greater surface area which caused presumably be due to cell division and cell-enlargement induced by application of GA [38] . It was further supported by findings of Sairam [39] , where the hormonal treatment, increased leaf area. Similar results have also been obtained by a few workers [40] - [42] on pre-sowing seed treatment and on foliar application [43] - [46] .
Moreover, the leaves of seed priming plants also exhibited a higher state of metabolic activity. It was evident by the elevated level for the PN (data not published) that might positively contribute to the enhanced LAI and plant dry mass production. The superior dry weight of the shoot and root of plant in response to GA treatment could be due to the cumulative effect of enhanced values of various growth parameters. The correlation studied also revealed that LAI has relationship with shoot and root dry weight with shoot length (data not published). Similar increase in dry matter production of sunflower due to application of pre-sowing seed treatment with GA has also been reported by [47] - [49] on sunflower and [40] [50] on other crops. The enhancement in CA activity and NR activity at both stages is greater at 10−5 M GA over control is a worth mentioning fact. The enzyme CA which catalyses the reversible hydration of CO2 to bicarbonate and maintains its constant supply to Rubisco case at the level of the grana of the chloroplast [51] - [55] . Moreover, CA is also known to be involved in diverse physiological processes such as photosynthetic electron transport [56] , and in maintain chloroplast pH during rapid changes in light intensity, ion-exchange, acid-base balance, carbohydrate/decarboxylation reactions and inorganic carbon diffusion between cell and its environment as well as inside the cell [57] .
Furthermore, the enhancing effect of GA on CA activity and NR activity may be attributed to the hormone induced increase in transcription and/or translation of the genes that code for CA [58] . This may also be attributed, as for growth characters, to its (GA) roles, on one hand, and compensation of the “hidden hunger” for GA by its pre-sowing seed treatment or foliar application on the other. These results corroborate the various other findings [59] [60] on sunflower and [61] [62] on other plant. The improvement in leaf N, P and K contents at both stages (50 and 70 DAS) resulting from pre-sowing seed treatment with GA particularly at 10−3 M GA over the control is not far to seek. These findings are in accordance with the results [20] [24] [26] .
Moreover, GA treatment enhancing the permeability of the membranes and absorption of nutrients which enhanced the vegetative growth and development of more seeds [21] [34] , and also due to existence of the imperative associators nutrients and biomass production [7] . It is a well-documented that a higher portion of leaf N is found in the chloroplast, most of it is invested in Rubisco alone, a key enzyme responsible for fixation of CO2 during the photosynthesis. Moreover, plants cultivated from GA-treated seeds (seed priming effect) exhibited higher exchange rate of H2O/CO2 through stomata. Wang et al. [13] suggested that this exchange rate of CO2/H2O may be controlled by mesophyll function. Therefore, a low hindrance against this CO2/H2O exchange rate, in turn, facilitates a free exchange of gases and a grater exchange rate for H2O and CO2 through stomatal pore. Further, the cumulative effect of increased CA and NR activities could have enhanced the availability of CO2 under a more efficient exchange of CO2/H2O along with its rate of reduction by Rubisco. The abortion of flowers and seeds is one of the important determining factors of plant productivity. Vegetative growth of a crop and physico-biochemical processes control the number and size of photosynthesizing sites are responsible for production of photosynthates (sucrose) even after flowering and their partitioning ultimately controls yield characteristics [7] [18] [23] . Now, needless to explain, efficient interaction of all these basic determinants, coupled with GA-generated increase in the cyclic and non-cyclic [9] , culminated in an increased rate of photosynthesis. Usually, GA is considered a promoter of photosynthesis and such results have also been reported by [34] . The increase in rate of photosynthesis implies more vegetative growth due to ample availability of nutrients. This, in turn, increases the size of the reproductive sink to attract more photo-assimilates and presumably results in greater potential for translocation of assimilates from the vegetative structures to pods. Sufficient availability of the assimilates subsequently leads to enhanced seed filling and culminates in increased seed yield, Table 11.
Increase in seeds per head suggesting the pre-sowing seed treatment of GA promotes differentiation leading to enhanced numbers of flowers, coupled with desirable development of under-developed seeds that result in stimulation of head-diameter, seeds per head and seed yield of treated plants, Table 11. It may also be added that application of GA promotes differentiation leading to enhanced number of flowers [8] [23] [56] . Its treatment may also be helpful in the desirable development of under-developed seeds particularly at the centre of the head as GA also causes cell division and cell enlargement [18] . These roles of GA directly or indirectly may be responsible for an increase in number of flowers coupled with the desirable development of under-developed seeds that result in higher values for head diameter and seeds per head of treated plants, Table 10.
Also, its promoting effect on PN, membrane permeability and transport of photosynthates may be helpful in favouring the partitioning of photosynthates towards developing seeds in the head, hence higher value for other yield related characters of treated plants. Thus, the higher values for vegetative, physio-biochemical and yield characters of treated plants may culminate in higher seed yield, Table 11. This proposition is further confirmed by correlation studies wherein seed yield has shown positive relationship with the various parameters studied, for example, at 50 and 70 DAS respectively, dry weight (![]() and 0.990), CA activity (
and 0.990), CA activity (![]() and 1.000), NR activity (
and 1.000), NR activity (![]() and 0.980), leaf N content (
and 0.980), leaf N content (![]() and 0.809), leaf P content (
and 0.809), leaf P content (![]() and 0.872) and leaf K content (
and 0.872) and leaf K content (![]() and 0.921) and at harvest, seeds per head
and 0.921) and at harvest, seeds per head![]() . These results are also in accordance with the findings of [43] [60] .
. These results are also in accordance with the findings of [43] [60] .
The increase in the above yield attributes may be traced to its various roles mentioned earlier leading to observed higher values for growth characters and, physiological and biochemical parameters of treated plants. Moreover, it mediates differentiation [21] [40] leading to enhanced number of flowers which develop into heads. As mentioned earlier, it plays role in cell division and cell enlargement [8] [16] [17] [21] resulting in proper development of under-developed seeds in heads, hence higher values for seed yield per plant weight of treated plants; PN [20] supplying sufficient C skeleton; and membrane permeability [19] and transport of photosynthates [22] [32] favouring partitioning, hence, higher values for the yield parameters of treated plants. Thus, higher values for growth, physio-biochemical and yield characteristics of treated plants may culminate in higher seed yield.
3.4. Effect of Seed-Priming Duration
The observed enhancement in the values for most of the growth characters, physiological and biochemical parameters and yield and quality characteristics at the various growth stages resulting from pre-sowing seed treatment for 8 h over 4 h is noteworthy. It may be added here that a specific concentration of a phytohormone like other metabolites is required for optimum performance of a plant. Pre-sowing seed treatment with GA for 4 h may not be sufficient for accumulation of the hormone inside the seeds at the specific level as the seed coat of sunflower is thick and hard. The specific level of the hormone might have been achieved by soaking the seeds for 8 h, hence higher values for most parameters studied. These results corroborate with the finding [40] [60] who also reported the effect of pre-soaking duration on the performance of brassica, black cumin and chickpea respectively.
4. Conclusion
The present study revealed that soaking of seeds in GA was more effective than the water soaked control for most of the parameters studied. The optimum concentration obtained for soaking the seeds in GA was 10−5 M. Duration of pre-soaking seed treatment with GA was also observed to be effective. Soaking the seeds in GA for 8 h was found to be optimum. Finally, it may be concluded that soaking the seeds with 10−5 M GA for 8 h is best for growth and development of sunflower cv. PAC 3776.
Acknowledgements
We are grateful to Professor L.N. Sharma and Associate Professor Dr. Khalil Khan for his critical comments and valuable suggestions with regard to the preparation of the manuscript.
NOTES
![]()
*Corresponding author.