Received 11 March 2016; accepted 19 April 2016; published 22 April 2016

1. Introduction
The increasing severity of environmental laws and the intense supervision of government environment agencies have forced many industries that still do not have adequate treatment of their waste to seek specific effluent treatment and to avoid the contamination of the air, soil and surface and ground waters. One example that can be cited is cobalt and its salts, considering that in the last century it was very little used industrially. Currently, in this century, the metal cobalt has been used in the production of super alloys [1] - [3] , while its salts have been principally used in catalysts [4] - [6] , fertilizers [7] - [9] , batteries [10] - [12] and ceramics [13] [14] .
Cobalt is a group VIII metal in the Periodic Table, bright grey in color and is present in small amounts in rocks, soil, water and plants. Generally, it is associated with other minerals, notably nickel, iron and copper.
Considering the use of metallic cobalt and of the salts in diverse industrial sectors, it is very likely that the effluent, if not effectively treated, can cause major environmental problems and damage to human health.
The Brazilian environmental agency (CONAMA) regulates the limit value of 25 mg/kg for disposal on the ground, while in water for human consumption the maximum allowed value is 0.05 mg/L [15] .
Work carried out by Chatterjee & Chatterjee [16] showed that an excess of cobalt in soil induces an iron deficiency, which can lead to a decrease in biomass, affecting photosynthesis and the development of catalytic activities within the soil.
In the case of the occupational health of workers in industries that operate with cobalt and its derivatives, it is recognized that the intense and continuous exposure to finely divided cobalt dust, powder or the absorption of oxides and salts (inorganic and organic) can result, directly or indirectly, in respiratory problems, allergic dermatitis, gastrointestinal effects (nausea, vomiting and diarrhea) and irreparable damage to the liver [17] [18] .
Due to the toxicity of cobalt along with its extensive use in industry, specific treatments are needed for its removal or to improve existing methods. This study therefore aims to present an electrolytic treatment that is able to remove efficiently the cobalt ions (Co2+) from effluents, contributing to the sustainability of the environment.
2. Materials and Methods
Initially solutions containing cobalt ions (Co2+) were prepared. These simulations were used as standardized effluent. All the solutions containing ions (Co2+) were made from cobalt sulfate heptahydrate (CoSO4・7H2O). The concentrations used in the experiments were 100, 200 and 400 mg Co+2/L. A 1 M sulfuric acid solution was also used to correct the pH value. The cathodes to be used in the electrolytic cell were made in a rectangular shape (4.5 × 4.0 cm) from a screen of galvanized carbon steel. The screen wire has a diameter of 0.30 mm and a gap of 0.55 mm mesh, as shown in Figure 1. The anode was made with a platinum plate having a surface area of 4.2 cm2.
In the experiments an electrolytic cell (Figure 2) constituted of an acrylic container with a maximum capacity of 490 mL was used, and it contained the anode (positive pole) and the cathode (negative pole) connected to a
![]()
Figure 1. Deposition of cobalt on the screen.
power supply of adjustable direct current (current rectifier) with a maximum voltage of 30 V. Continuous agitation of the solution was performed with a magnetic stirrer (magnetic bar enveloped in Teflon) in order to increase the release of hydrogen (H2) on the surface of the screen.
Some of the operational conditions, such as the time and current/voltage for this experiment, were based on Faraday's law (Equation (1)). This equation requires that: a) the amount of substance deposited is directly proportional to the amount of electricity passed through the electrolytic solution; b) the quantities of different substances are deposited in proportion to their electrochemical equivalents. Thus, using Equation (1) [19] , it is possible to estimate the time required to deposit the metal on the carbon steel cathode.
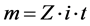 (1)
(1)
where:
m = mass, g;
Z = electrochemical equivalent (g/ coulomb);
i = current, A;
t = time, h.
Using this equation for cobalt, it is possible to calculate the theoretical amount of metal that can be deposited on the cathode in an experiment. As the objective was for all cobalt ions (Co2+) to be removed from the various solutions under the predetermined conditions of operation, in this way, it is possible to choose the variable of interest over time.
After executing the removal experiments, the residual concentrations of cobalt ions (Co2+) in each sample were checked using a voltammetric analyser (VA 767 Metrohm Computrace). This equipment works with three electrodes combined: the working electrode (mercury multimode), reference electrode (Ag/AgCl-KCl 3.0 mole/L) and an auxiliary platinum electrode. In the preparation of the standard solution of Co2+, ultrapure water and cobalt sulfate heptahydrate (CoSO4∙7 H2O) were used.
The removal efficiencies were then calculated using Equation (2).
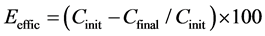 (2)
(2)
where:
Eeffic = efficiency of removal (%);
Cinit = concentration of solution before the electrochemical removal process;
Cfinal = concentration of the solution after the electrochemical removal process.
Based on the efficiency of removal, the influence of the following parameters was evaluated: pH, concentration of cobalt ions, current application in the cathode and the exposure time.
3. Results and Discussion
3.1. Influence of pH Value on Removal of Co2+
The graph presented in Figure 3 shows the removal efficiency of Co2+ with variation of the pH value considering the current fixed at 0.3 A, the time of 15 minutes and the concentration of Co2+ in 200 mg/L.
It turns out that under the conditions laid down for pH = 4.0, a 43% removal of Co ions is achieved. However, as the value of the pH decreases there is an intense hydrogen evolution, resulting in active competition with the Co2+ ions and reducing the effluent removal. Then, as the hydrogen overvoltage on the cobalt is small, the efficiency of Co2+ is closely related to the pH of the solution. The reactions that favor the evolution of hydrogen are: 2 H+ + 2e ® H2 and 2H2O + 2e ® H2 + 2OH-. With the decrease of pH, the evolution of hydrogen is made easier [20] [21] .
3.2. Influence of Current on Removal of Co2+
To evaluate the effect of the current in the process, solutions with concentrations of 200 mg/L and chains ranging from 0.1 to 0.5 A were used. The time for removal was maintained at 15 minutes with the initial pH of 4.0. From Figure 4, the removal efficiency as a function of current applied to the system can be seen, thus obtaining a better result for a current of 0.3 A, with 43% removal efficiency.
![]()
Figure 3. Influence of pH on removal of Co2+.
![]()
Figure 4. Influence of current on removal of Co2+.
3.3. Influence of Initial Concentration of Co2+ and Time on the Removal Process
Figure 5 shows the removal efficiency for three different initial concentrations of Co2+ (100, 200 and 400 mg/L), in experiments whose time ranged from 15 to 185 min. The initial pH was maintained at 4.0 and the current was set at 0.3 A.
The results show a significant increase in removal efficiency over time for all the initial concentrations evaluated. This can be explained by Faraday’s law for electrolytic cells, in which the equation m= k.i.t demonstrates that the mass of deposited metal is directly proportional to the time (t) and intensity of current (i) applied [19] .
For the initial concentration of 400 mg/L, there is an increase in the removal efficiency of 28% after 15 minutes and 73% after 185 minutes. The removal efficiency was 70% for the time of 165 minutes, demonstrating little gain above this time, and indicating a possible saturation of the screen. It may be said that the smaller the amount of Co2+ ions in the solution, the greater will therefore be the hydrogen evolution (H2), competing with the deposition of the Co. At 200 mg/L, the removal efficiency increases from 43% to 84% for the same time interval, and from 135 min (80% of removal) screen saturation occurs. At 100 mg/L, the increase is more significant, rising from 6% to 88% in the same time interval, and saturation occurs from 135 min.
Another issue that must be stressed is the evolution of hydrogen during the process. This was constant and probably decisive in decreasing the removal efficiency. Agitation was applied to minimize the consequences of this evolution.
![]()
Figure 5. Influence of initial concentration of Co2+ and time on the removal process.
4. Conclusions
Based on the literature and laboratory tests, the following conclusions are made:
Considering the toxicity of cobalt in the form of the metal or its compounds (inorganic and organic), the monitoring of effluents and development of the removal process is essential;
An electrochemical process is a simple and effective way to remove cobalt ions from effluents;
The electrolytic cell used in the experiments showed itself to be capable of promoting the removal of cobalt ions from simulated wastewater;
The galvanized carbon steel cathode screen proved to be cheap, simple and efficient to use in the electrolytic cell;
The results of electrolytic tests for cobalt ions (Co2+) from aqueous effluent, in standardized conditions (current of 0.30 A, a voltage of 30 V, and a time of removal of 185 minutes), were very satisfactory. Electrolytic removal efficiency using solution concentration of 400, 200 and 100 of mg Co2+/L was, respectively, 73%, 84% and 88%.