Received 4 November 2015; accepted 25 December 2015; published 28 December 2015

1. Introduction
Carbon dioxide CO2 is one of man-made greenhouse gases that are emitted by combustion of fossil fuels, such as coal, oil, and natural gas. Carbon dioxide is emitted from many power plants for generating electricity, power vehicles, heat homes, cook food and much more. However, fossil fuels are essentially a non-renewable energy source [1] . Therefore, the amount of fossil fuel reserves will be diminished in future by consumption and the cost of finding and extracting new underground resources will be much more expensive for everyday use [2] [3] . It might be also serious that CO2 would cause global warming by absorbing and emitting radiation within the infrared range.
Therefore, the reduction of emission of carbon dioxide and the reduction of consumption of fossil fuels are crucial subject that must be settled urgently.
In order to suppress the emission of CO2 into the environment from a power plant, for example, it might be desirable that CO2 is collected before exhausting and converted to methane, if any surplus renewable electric power exists. This means that surplus electric energy can be converted to chemical bonding energy of methane. That is, the surplus electric energy is stored as methane [4] - [6] . This method is superior to batteries, because the electric energy stored in batteries will be gradually lost by natural discharge. However, the energy stored in methane will be conserved for a long time without significant loss [7] .
In order to reduce CO2 with hydrogen various experiments were carried out by using discharge system [8] - [13] . In most cases, CO2 was reduced by CH4 to form syngas of CO and H2 [14] - [20] , because methane is also one of the greenhouse gases. Eliasson et al. investigated the production of CH4 by a dielectric barrier discharge with H2 in detail [8] . However, a new innovative method for improving CO2 decomposition, CH4 selectivity, and energy efficiency has been expected.
The purpose of this study is to investigate fundamental process of reduction of carbon dioxide to generate beneficial and reusable organic materials like methane by using low-pressure discharges [21] [22] . We here employed a low pressure discharge under the magnetic field. As well known, the discharge under atmospheric pressure was a very narrow streamer type. On the other hand, the discharge in low pressure was spatially spread and it was easy to obtain a non-equilibrium plasma, where the dissociation and decomposition effectively occurred by the electrons. In order to improve the reaction rate, the electron energy and density are quite important. Especially, in the low pressure, the magnetic field is useful to confine electrons to increase the electron density. The purpose of this experiment was to clarify the effect of discharge power on the formation of CH4 by the reduction of CO2 in low pressure discharge with hydrogen under the magnetic field.
2. Experimental Apparatus
Figure 1(a) shows a schematic of the experimental apparatus consisting of a coaxial cylindrical system [23] . Mixed gas of carbon dioxide and hydrogen was fed to the discharge chamber made of glass tube. The gas-mix- ing ratio and total flow rate were controlled by mass flow controllers, independently. Total pressure was adjusted by a needle valve and fixed at 200 Pa. Here, we employed a negative square-pulse voltage Vp with a pulse duration of 5 μs that was supplied to a small electrode. Repetition frequency of the square pulse was fixed at 1.25 kHz through the whole experiment. The gas passing through the discharge region was evacuated by a rotary pomp. Fourier transform infrared spectroscopy (FTIR) was employed to analyze the gas species before and after
![]()
Figure 1. Schematic of the experimental setup. (a) Whole experimental system with inlet of mixed gas CO2/H2 and outlet to FTIR and rotary pomp. Pressure is adjusted by a needle valve. Details in a broken circle is shown in (b). A rod electrode is installed inside a pipe magnet (with polarity S and N) (left hand side), and a rod magnet is installed inside a grounded stainless pipe electrode (right hand side). d denotes electrode distance (=2 - 3 mm). Gas flow direction is indicated by white arrows. Directions of crossed magnetic and electric fields denoted by B and E in the discharge region are indicated by dotted and solid arrows, respectively.
the discharge [21] [22] . For the measurement of small amounts of gas species, the sampled gas was introduced into FTIR with a gas cell with multiple laser reflection system, where precise absolute values of each gas species were possible to measure by comparing with typical FTIR signal database.
In order to proceed a discharge under the magnetic field, two kinds of permanent magnets were employed as shown in Figure 1(b). One was a pipe magnet (17 mm long, 4 mm inner diameter, and 9 mm outer diameter) made of samarium cobalt, the magnetic strength of which was 0.4T at the end surface. The other one was a rod magnet (27 mm long, 4 mm diameter) made of samarium cobalt, the magnetic strength of which was also 0.4T at the end surface. Inside the pipe magnet a powered electrode of 2 mm in diameter was inserted for the discharge from the left-hand side as shown in Figure 1(b). The grounded electrode was a stainless pipe (50 mm long, 6 mm inner diameter, and 8 mm outer diameter), inside of which the rod magnet was installed. In order to avoid direct contact between the plasma and the magnets, thin mica plate of 0.5 mm thick was attached to the end surface of the magnets. The tip of the powered electrode protruded 1 mm from the mica plate. The entire electrode system was installed inside the glass tube of 10 mm in inner diameter and 12 mm in outer diameter. Gas flow direction was indicated by thick white arrows in Figure 1(b).
Since the magnetic field and the electric field supplied by the magnets and powered electrode, respectively, intersect with an oblique angle each other, as shown in Figure 1(b), a cross field discharge, i.e., a glow discharge with a perpendicular component of magnetic field, can be realized. The discharge under the magnetic field has an advantage for increasing the electron density in a core plasma region and reducing the electron diffusion across the magnetic field toward downstream region. Owing to the electron density increase, dissociation of CO2 and H2 will be enhanced. On the other hand, suppression of electron diffusion toward downstream region will reduce re-decomposition of CH4 by electrons. As a result, an enhancement of CH4 yield is expected.
3. Experimental Results and Discussions
3.1. Experimental Conditions
The degree of electron magnetization was evaluated from ωceτcn ~ 7.5 for hydrogen at 200 Pa. Here, ωce is electron cyclotron angular frequency at the magnetic field of B = 0.4 T and τcn is collision time of electrons with neutral hydrogen. Since ωceτcn was much larger than 1, the electrons were sufficiently magnetized.
Variations of the magnetic field strength supplied by the pipe magnet and the rod magnet along the center axis are plotted in Figure 2 by sold and dotted curves, respectively, as a function of the distance from the surface of the powered electrode. Actual magnetic field strength at the position between the electrodes can be obtained by a summation of both components of magnetic field. Therefore, the magnetic field of 0.45 - 0.55 T was applied in the discharge space. From this configuration we could investigate hydrogenation of CO2 to CH4 under magnetic field.
The results were evaluated by the following quantities.
(i) CO2 decomposition ratio α (%):
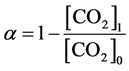 (1)
(1)
![]()
Figure 2. Variations of magnetic field strength, supplied by a pipe magnet (solid curve) and a rod magnet (dotted curve), along the center axis of a glass tube in the region indicated by a double-headed arrow in Figure 1. The distance was counted from the surface of powered electrode. The electrode distance was 2 mm.
(ii) CH4 selectivity β (%):
 (2)
(2)
(iii) Energy efficiency γ (L/kWh) for CH4 production:
 (3)
(3)
Here, [a] denotes amount of a, and suffix 0 and 1 correspond to the values before and after the discharge, respectively. These quantities show how much carbon in CO2 has been converted to methane. γ is also an important factor to realize a suitable commercial system for producing methane in high efficiency. Discharge power was obtained by a time averaged value of V(t)I(t) supplied from the pulse generator, where V(t) and I(t) were applied voltage and discharge current at time t, respectively. Here, 1 L/kWh corresponds to 44.6 mmol/kWh (=0.71 g(CH4)/kWh). Energy efficiency can be also expressed by γ = αβГ/100Pin, where Γ is input partial flow rate of CO2 and Pin is input electric power for the discharge.
Gas species measured by FTIR showed that main carbon products were CH4 and CO through the whole experiment. Here, CO might come from the following dissociation reaction by electron impact collisions; e + CO2 → CO + O. Hydrocarbon species was only CH4, and the other COH2 and CH3OH were not detected. We could not detect other organic materials such as ethane, ethylene, and acetylene. But, the production of steam H2O was detected. Therefore, it was shown that methane was actually only a hydrocarbon produced from CO2. Therefore, in this case, methane selectivity β could be simply expressed by β = [CH4]/([CH4] + [CO]). Through the experiment we fixed pulse repetition frequency of applied pulse voltage at 1.25 kHz. Flow rate of CO2 was also fixed at 1 sccm (standard cubic centimetres).
3.2. H2 Flow Rate Dependence
Dependencies of CO2 decomposition ratio α, CH4 selectivity β, and energy efficiency γ on H2 flow rate were briefly reported in Ref. [23] with and without magnetic field. The discharge power was set at 30 W. When the magnetic field was introduced, the maximum β of 27% was obtained at H2 = 10 sccm. This value was higher than that of about 16% in the case without magnetic field. It was found that γ in the case with magnetic field raised almost 3 times as much as that in the case without magnetic field. The maximum value of γ attained to about 0.46 L/kWh when H2 flow rate was about 10 sccm.
In our system α was not much changed by the hydrogen flow rate. However, β has a strong dependence on H2 flow rate in the regime below 10 sccm [23] . The maximum of β attained to about 27% at H2 = 10 sccm when the cross field discharge was employed. In the lower H2 flow rate regime the reaction was determined by amount of H2 supplied. The production of CH4 was increased with an increase in H2 flow rate, according to the following reaction.
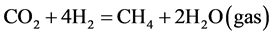 (4)
(4)
Here, taking account of a simultaneous production of CO it was reasonable that optimum amount of H2 for CH4 production exceeded stoichiometry value of 4 sccm, i.e., CO2:H2 = 1:4 as in reaction (4). When H2 flow rate was much more increased, transit time of H2 gas flow within the discharge region was decreased, which might result in a decrease in CH4 production.
3.3. Input Power Dependence
In our system, permanent magnets were directly attached to the electrode via a thin mica insulator sheet of 0.5 mm thick, in order to avoid heat damage and to keep electrical insulation. Therefore, a change of the electrode distance d between the powered electrode and the grounded pipe electrode also brought about a change of the strength of magnetic field B in the discharge region. That is, when d was changed from 2 mm to 3 mm, the strength of magnetic field at the middle point on the center axis between the electrodes was varied from 0.45 T [= 0.225 T (dotted curve) + 0.225 T (solid curve)] to 0.335 T [= 0.175 T (dotted curve) + 0.16 T (solid curve)] (see Figure 2).
Input power Pin dependencies of α were plotted in Figure 3 with the electrode distance d as a parameter. Here, flow rate ratio was kept at CO2:H2 = 1 sccm:4 sccm. With increasing input power, CO2 decomposition ratio β increased and attained to 25% at Pin = 40 W when d = 2 mm. Then, the decomposition ratio β was gradually saturated and approached to 35% when Pin was further increased to 160 W. One of the reasons for this saturation was an input power loss by a heating of the electrodes, rather than a production of plasma. It was also noted that a small gap distance between the electrodes was effective for the decomposition of CO2. This might be due to an increase of the electric field for the plasma production and a resultant increase of input power density for CO2 decomposition, when the electrode distance d was changed from 3 mm to 2 mm.
Similar dependency was observed for CH4 selectivity β as shown in Figure 4. In the case of d = 2 mm, β increased almost in proportion to input power in the range Pin < 30 W. We got β = 30% at Pin = 30 W. However, the increment rate of β was reduced in the range Pin > 30 W. Even in this case, however, β attained to 35% when Pin = 130 W. It was also found that the input power for getting the same value of β was reduced by decreasing d from 3 mm to 2 mm. We got β = 30% when Pin was increased up to 50 W.
Production rate of methane can be obtained from the product αβГ/100. Here, Г (=1 sccm) is the initial flow rate of CO2. Figure 5 shows the production ratio of CH4from CO2as a function of input power with d as a parameter. Vertical axis also shows how much percent of CO2 is converted to CH4via a reaction with H2. With an increase in input power Pin, the production of CH4 was increased almost in proportion to input power. However, CH4production was saturated above 50 W, and finally about 15% of CO2 was converted to CH4 at Pin = 160 W
![]()
Figure 3. Variation of CO2 decomposition ratio α as a function of input electric power with electrode distance d as a parameter in the case with magnetic field. Flow rate ratio is CO2:H2 = 1 sccm:4 sccm.
![]()
Figure 4. Variation of CH4 selectivity β as a function of input electric power with d as a parameter in the case with permanent magnets. Flow rate ratio is CO2:H2 = 1 sccm:4 sccm.
![]()
Figure 5. Production ratio of CH4 from CO2 as a function of input power with d as a parameter in the case with permanent magnets. Vertical axis shows how much percent of CO2 is converted to CH4. Flow rate ratio was CO2:H2 = 1 sccm:4 sccm.
in the case of d = 2 mm. Production efficiency in the case of d = 2 cm is roughly two times as much as that in the case of d = 3 cm.
Finally, power dependence of energy efficiency γ was plotted in Figure 6 with d as a parameter. We got maximum γ of 0.53 L/kWh when Pin = 30 W and d = 2 mm. Although β and β increased with input power, γ was conversely reduced to about a half at Pin = 160 W. Therefore, the total energy efficiency was fairly reduced in the high power regime. It was noted that γ became low in the overall power regime when d = 3 mm, compared to the case of d = 2 mm. This might be due to a decrease of input power density for the plasma production as mentioned above.
The results shown in Figure 3 and Figure 4 were replotted in Figure 7(a) and Figure 7(b), respectively, as a function of power density (W/mm3) with the electrode distance d as a parameter. Here, power density was obtained from input power (W) divided by the discharge space volume (mm3). The decomposition ratio α seemed to be simply dependent on the input power density (W/mm3), rather than d, as shown in Figure 7(a). The value α increased with an increase in power density and finally reached 25% at 0.2 W/mm3. Then, β was gradually saturated in the higher power density range (>0.2 W/mm3). Similar dependence was also found for the CH4 selectivity β. The dependence of β on power density seemed to be nearly independent of d, but dependent on power density as shown in Figure 7(b). The value of β was abruptly increased with an increase in input power density below 0.2 W/mm3, and finally saturated around 30% at about 0.2 W/mm3. Then, β gradually increased in the higher power density regime (>0.2 W/mm3).
![]()
Figure 6. Variation of energy efficiency γ as a function of input electric power with d as a parameter in the case with permanent magnets. Flow rate ratio was CO2:H2 = 1 sccm:4 sccm.
![]()
Figure 7. Variation of CO2 decomposition ratio α and CH4 selectivity β as a function of input electric power density with d as a parameter in the case with permanent magnets. Flow rate ratio was CO2:H2 = 1 sccm:4 sccm.
4. Discussion
Discharge within a very narrow gap of 2 mm in the presence of magnetic field was quite effective for conversion from CO2 to methane. Under the magnetic field, electrons are magnetized and confined for a long time in the discharge. Owing to these electrons, mixed gas of CO2 and H2 was decomposed in the discharge and finally CH4 was produced in the downstream region through a recombination of decomposed radicals. The decomposition ratio was expectedly increased in a plasma with magnetic field.
Figure 8 schematically shows a model for CH4 production in the cases (a) without and (b) with the magnetic field. As shown in Figure 8(a), when no magnetic field is applied, the electrons produced between the electrodes diffuse toward the downstream region as shown by thick arrows, with radicals CO*, H2*, and H*, which are mainly produced by electron impact collisions in a core plasma, i.e., e + CO2 → CO* + O* and e + H2 → H2* → H* + H*. Eventually, these radicals react with each other to produce CH4 in the downstream region. It should be noted that, during this synthesis process, however, CH4 produced would be simultaneously re-decomposed by the electrons through the following electron impact dissociation collisions, i.e., e + CH4 → CH2* + H2*. Then, total amount of CH4 production would be suppressed during the diffusion in the presence of elections as in a core plasma region between the electrodes. Therefore, CH4 selectivity β would be reduced.
On the other hand, when the magnetic field is introduced, magnetized electrons are suppressed to diffuse across the magnetic field toward the downstream recombination reaction region. On the other hand, neutral radicals, which are not magnetized, are able to diffuse toward the downstream region as shown by white arrows in Figure 8(b). Then, CH4 synthesis proceeds among those radicals in such electron-suppressed downstream space, where simultaneous re-decomposition of CH4 by electron impact dissociation collisions were reduced. Then, CH4 selectivity β would be improved.
Further, since the magnetic field can confine the electrons, electron density in the core plasma region increases. Then, CO2 decomposition ratio β is also increased by the following dissociative collisions with electrons, i.e., e + CO2 → CO* + O*. As a result of the increases in α and β, energy efficiency γ could be markedly increased in the case with the magnetic field, as shown in Figure 6.
As shown in Figure 7, α and β are a function of power density, increasing with power density. This result can explain the results in Figure 6. That is, when the input power is fixed, the power density in the case of d = 2 mm can be higher than that of d = 3 mm. Therefore, both β and β in the case of d = 2 mm would be higher than those in the case of d = 3 mm at fixed input power. Therefore, since γ ~ β, a large γ can be obtained when d = 2 mm. Actually, energy efficiency γ attained to 0.53 L/kWh, which was rather higher than those of the previous magnetic-field free experiments [8] [9] . The use of magnetic field is quite effective for a higher rate conversion of CO2 to CH4.
![]()
Figure 8. A model of CH4 synthesis in (a) absence and (b) presence of magnetic field (dotted line). In (a), both electrons and neutral radicals (CO*, H2*) diffuse toward downstream recombination region, where CH4 is formed with an effect of re-decomposition by electrons. In (b), diffusion of magnetized electrons across the magnetic field is suppressed. CH4 synthesis proceeds with less effect of re-decomposition by electrons.
The energy efficiency γ described above did not include the energy for generating H2 from the water, for example. Electrolysis is a promising option for hydrogen production using renewable resources. Industrial electrolyzer has a nominal hydrogen production efficiency of around 73% [24] . Further, a maximum efficiency value of 96% was reported for the case of low carbon steel electrodes, which was much higher than 73%, the average efficiency of common commercial and industrial electrolyzers [25] . Here, we concentrated only on the energy efficiency for the production of CH4 from CO2 with a use of given H2 and CO2.
Finally, we discuss a carbon balance. As mentioned above, the materials containing carbon, produced by the discharge, were simply CH4 and CO. Methanol was scarcely produced. The other carbon materials such as COH2 and C2 hydrocarbons like ethane, ethylene, and acetylene were not detected. Carbon film deposition was also not observed in a lower power regime (<30 W) when [H2]/[CO2] ? 1. This would be due to the fact that carbon atom might be immediately either oxidised by oxygen or reduced by hydrogen when carbon atoms were produced in the discharge. The resultant carbon balance was simply expressed as follows:
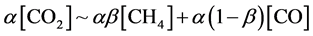
As described above, in our experiment, CO2 and H2 were decomposed to form CO*, H2* and H* by electrons in the plasma. Then, CO* was reduced by H2* and H*, and finally CH4 was produced. This process was quite similar to Sabatier reaction [26] , where CO2 was dissociated to CO* → C* + O* on a heated Ni surface. Then, H2 reacted with C* and O* on Ni surface to generate CH4 [27] [28] . In our case, the electrons in the plasma played a similar role as a catalysis effect of Ni.
It should be also noted that γ in our discharge system was much higher than that of conventional magnetic-field free discharges. The energy efficiency in the case of high-pressure dielectric-barrier discharge (DBD) was reported to be 0.06 L/kWh, where β = 12.4%, β = 3.2% for the total flow rate of Γ = 500 sccm (CO2:H2 = 1:3) and input power of 500 W [8] . For a low pressure microwave discharge, γ = 0.027 L/kWh was reported with β = 81% and β = 1.2% at input power of 3 kW [9] . Therefore, energy efficiency in our case was about 20 times as much as that in the low-pressure microwave case.
5. Conclusion
Methane was produced from carbon dioxide in a low-pressure square-pulse cross-field discharge with hydrogen. Methane was only organic species produced from CO2. Only CO was detected as non-organic by-product. We found that the decomposition ratio β of CO2, methane selectivity β, and energy efficiency γ in a short electrode distance under the magnetic field were superior to those of the magnetic-field free cases. We obtained β = 25%, β = 30%, and γ = 0.53 L/kWh under optimized condition at flow rate ratio of CO2:H2 = 1:4, electrode distance of d = 2 mm, and input power of Pin = 30 W under the magnetic field of 0.45 T. Therefore, the discharge in a small volume with magnetic field was effective for an efficient production of CH4 from CO2 in a low pressure CO2/H2 discharge plasma.