1. Introduction
HIV/AIDS epidemic continues to be the main killer disease in sub-Saharan Africa, with majority of infections and deaths occurring in adults. In 2013, the Joint United Nations Programme on HIV/AIDS (UNAIDS) and the World Health Organization (WHO) estimated that 35 million people were living with HIV in the world, 2.1 million were newly infected, and 1.5 million deaths occurred. Of these, 24.7 million lived in sub-Saharan Africa with 1.5 million new infections and 1.1 million AIDS deaths occurring in the same year [1] .
HIV transmission is not uniform between countries and among different stages of the disease within an infected individual [2] - [4] . As an infected person progresses from one stage of HIV infection to another, the level of transmitting the viruses to others changes. The transmission dynamics of the virus can be categorized into three stages of HIV progression of an infected individual [5] [6] . The first stage is the primary stage which follows soon after the initial infection. The second stage is the asymptomatic stage. In this stage, symptoms are few, and the patient’s blood contains a relatively small viral load and antibodies to the virus. The last stage is the symptomatic or AIDS stage. In this stage, an infected individual stays for a period of about 1 - 2 years until death, if no treatment is used.
Viral load varies greatly between these stages. During the period of primary infection, viral load is typically high. The viral load drops as one enters the asymptomatic period, followed by a symptomatic/AIDS stage during which the viral load is extremely high. A community-based study in which couples are prospectively followed for 30 months to evaluate the risk of transmission in relation to viral load and other characteristics shows that the risk of infection increases as an infected person’s viral load increases [2] . Another study also shows that the level of HIV infection is dependent on clinical status of the individual [7] . Most transmissions occur shortly after infection, after which infections become low until the immune system begins to be seriously affected.
The study of an epidemic, such as HIV, and its spread process in any community, is different to investigations in many other sciences. Data cannot be obtained through experiments in the population, but can only be obtained from surveys and results found in published or unpublished documents. These data are often not complete, may be inaccurate, and may vary with respect to methods used to collect them. Due to difficulties in obtaining HIV data, mathematical modelling and numerical simulation play an important role in analysing the behaviour of the epidemic, measuring its past, present, and future effect in a society.
Variations in the level of HIV transmission over time can have an impact on the estimates delivered from mathematical models. Existing models of transmission have either assumed a single group of infected individuals or have included stages of HIV progression to capture the transmission dynamics but have not studied how these two modelling approaches differ from each other. Lin et al. [8] studied a model that included stages of HIV progression in a general way. In a similar manner, McCluskey [9] [10] studied a similar model which was extended to include the effects of treatment. In all these models, none of them investigates the impact of including stages in the model and how the differences in the transmission among stages can have an impact on the results. In this work, the impact of including stages of HIV progression in models is investigated in order to understand how the model with and without stages affect the estimates produced.
2. Simple HIV Model
The HIV/AIDS model formulated here considers the total population, 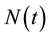 in a single homogeneous group divided into two subgroups: the susceptible population,
in a single homogeneous group divided into two subgroups: the susceptible population, 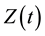 , and infected individuals,
, and infected individuals,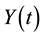 . The demographics of the model are described by the rates of entry and exit of individuals from the population. The parameter b is the rate at which new individuals are recruited into the susceptible group, μ is the natural mortality rate (
. The demographics of the model are described by the rates of entry and exit of individuals from the population. The parameter b is the rate at which new individuals are recruited into the susceptible group, μ is the natural mortality rate (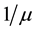 is the life expectancy of individuals), and
is the life expectancy of individuals), and 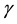 is the rate at which infected individuals die from the disease.
is the rate at which infected individuals die from the disease.
The dynamics of the model are governed by the following system of differential equations:
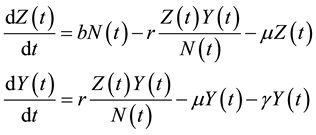 (1)
(1)
where, r is the transmission rate and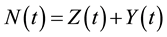 . From the above model, the total population change according to:
. From the above model, the total population change according to:
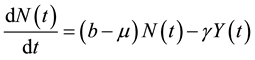 (2)
(2)
In this case, the total populations varies. This can be due to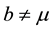 , or
, or 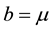 and
and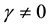 . All parameters of the model are positive. The system is epidemiologically and mathematically well-posed in the sense that, if the initial data
. All parameters of the model are positive. The system is epidemiologically and mathematically well-posed in the sense that, if the initial data 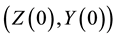 is a positive region in two dimension, the solutions remain defined for all
is a positive region in two dimension, the solutions remain defined for all 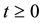 in the same region.
in the same region.
Because 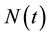 varies over time, steady states are not expected in any parts of the population, but they may occur for the proportions. The model in Equation (1) is therefore converted into proportions. We define
varies over time, steady states are not expected in any parts of the population, but they may occur for the proportions. The model in Equation (1) is therefore converted into proportions. We define 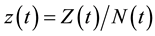 and
and 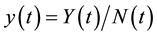 to obtain:
to obtain:
![]() (3)
(3)
which is positively invariant in the region:
![]()
Note that Equation (2) can be re-written as:
![]() (4)
(4)
which integrates to:
![]() (5)
(5)
indicating that when![]() , the dynamics of the total population is strongly governed by the proportions of infected individuals in the population.
, the dynamics of the total population is strongly governed by the proportions of infected individuals in the population.
2.1. Existence of the Equilibria
This section derives stability conditions of the equilibrium points of the system in Equation (3).
Definition 2.1. Given a system of differential equations![]() , an equilibrium point of this system is a point in the state space for which
, an equilibrium point of this system is a point in the state space for which ![]() is a solution for all t.
is a solution for all t.
We define a threshold factor:
![]() (6)
(6)
from Equation (1) to represent the average number of secondary infections caused by one infective individual introduced into a completely susceptible population.
Theorem 2.2. For r > γ, the system in (3) always has a disease free equilibrium ![]() if
if ![]() and a unique endemic equilibrium point
and a unique endemic equilibrium point ![]() with
with ![]() and
and ![]() exist only if
exist only if![]() .
.
Proof. From the second equation of (3), with the right hand side equal to zero at large t, then equilibrium points must satisfy:
![]() (7)
(7)
or
![]() (8)
(8)
and
![]() (9)
(9)
Substituting (7) and (8) in (9) or in the first equation of (3) with the right hand side equal to zero, gives ![]() or
or ![]() respectively. Equation (6) can be rewritten as
respectively. Equation (6) can be rewritten as![]() . Substituting it in (8) gives
. Substituting it in (8) gives![]() . If
. If![]() , the only equilibrium in the region D is
, the only equilibrium in the region D is![]() ; if
; if![]() , the only equilibrium in D is
, the only equilibrium in D is![]() . Therefore, the model has only two equilibrium points.
. Therefore, the model has only two equilibrium points.
2.2. Stability Analysis
Local stability of the equilibrium points is performed by introducing of small perturbations, ![]() ,
, ![]() at the equilibrium points as:
at the equilibrium points as:
![]()
![]()
and substituting them in Equation (3). Because ![]() and
and ![]() are very small quantities, we discarding terms of higher order to obtain:
are very small quantities, we discarding terms of higher order to obtain:
![]() (10)
(10)
The coefficients of the perturbations give the Jacobian matrix:
![]() . (11)
. (11)
At the disease free equilibrium, the Jacobian matrix in (11) gives the following characteristic equation:
![]() (12)
(12)
with eigenvalues ![]() and
and![]() . If
. If![]() , both eigenvalues are real and negative and χ < 1. The disease free equilibrium become stable. If
, both eigenvalues are real and negative and χ < 1. The disease free equilibrium become stable. If![]() , then
, then ![]() and the two eigenvalues have opposite signs. In this case, one solution
and the two eigenvalues have opposite signs. In this case, one solution ![]() approaches the equilibrium while the other
approaches the equilibrium while the other ![]() moves away. The equilibrium point is therefore unstable.
moves away. The equilibrium point is therefore unstable.
Substituting the endemic equilibrium point, the Jacobian matrix ![]() gives the characteristic equation:
gives the characteristic equation:
![]() (13)
(13)
where,
![]() (14)
(14)
![]() (15)
(15)
![]() (16)
(16)
and
![]()
If![]() , it implies that
, it implies that![]() . Therefore, the trace of the matrix,
. Therefore, the trace of the matrix, ![]() because
because
![]()
and the determinant, ![]() because
because
![]()
Thus, by the Routh-Hurwitz criterion, all of the eigenvalues have negative real parts and the endemic equilibrium point is stable. On the other hand, if![]() , then
, then ![]() and
and ![]() while
while![]() , making one of the eigenvalue have a positive real part. Therefore, the endemic equilibrium point is unstable.
, making one of the eigenvalue have a positive real part. Therefore, the endemic equilibrium point is unstable.
3. HIV Model with Stages
Here, the model in (0) is extended to include stages of HIV progression. The infected group is divided into two subgroups: those in the primary stage of HIV infection, ![]() and those in the asymptomatic stage,
and those in the asymptomatic stage,![]() . The rate of progression from
. The rate of progression from ![]() to
to ![]() is
is ![]() (assuming a constant rate) and
(assuming a constant rate) and ![]() is the time an infected individual spends in the primary stage (waiting times). Infected individuals in
is the time an infected individual spends in the primary stage (waiting times). Infected individuals in ![]() die at a rate
die at a rate![]() . The dynamics are governed by the system:
. The dynamics are governed by the system:
![]() . (17)
. (17)
From the above model, the total population at time t is given by:
![]() (18)
(18)
The parameters ![]() and
and ![]() determine transmission rates due the interaction between the susceptible individuals,
determine transmission rates due the interaction between the susceptible individuals, ![]() and infected individuals in subgroups
and infected individuals in subgroups ![]() and
and![]() , respectively. A study by Quinn et al [2] showed that transmission of the viruses from individuals in the primary stage to the individuals in the susceptible group is higher than those in the later stages. Therefore,
, respectively. A study by Quinn et al [2] showed that transmission of the viruses from individuals in the primary stage to the individuals in the susceptible group is higher than those in the later stages. Therefore, ![]() in this model.
in this model.
For stability analysis, the system in (17) is converted into proportions by letting![]() ,
, ![]() , and
, and![]() . Thus,
. Thus,
![]() (19)
(19)
where
![]() (20)
(20)
integrating to
![]() (21)
(21)
The dynamic behaviour of the total population in this model is mainly governed by infected individuals who are in the asymptomatic stage of HIV infection,![]() . This is because, it is only in this group individuals die from the disease.
. This is because, it is only in this group individuals die from the disease.
Model (17) then becomes:
![]() (22)
(22)
with
![]() (23)
(23)
The above system have a positively invariant feasible region given by:
![]() (24)
(24)
with all parameters positive.
The incidence of the disease is the proportion of new cases occurring in a population during a defined time interval. Using this model, incidence is given by:
![]() (25)
(25)
where I is the incidence, and ![]() is the average time spent in the primary stage given by:
is the average time spent in the primary stage given by:
![]() (26)
(26)
The prevalence of the disease is defined as the proportion of infected individuals in a population. From the model, prevalence is simply infected individuals in![]() .
.
3.1. Existence of the Equilibria
Using the Next-generation technique [11] , the threshold quantity of the model is given by:
![]() (27)
(27)
which is a linear combination of the threshold quantities of the infected individuals in the primary stage, ![]() and in the asymptomatic stage,
and in the asymptomatic stage,![]() . A factor
. A factor ![]() is the probability that an infective individual will leave the primary stage of infection and enter the next stage (the asymptomatic stage).
is the probability that an infective individual will leave the primary stage of infection and enter the next stage (the asymptomatic stage).
Because of variable population size, system (22) is complex and calculation of the endemic equilibrium points is difficult. The dynamic behaviour of the population size (Equation (21)) is considered.
Definition 3.3. As![]() , an equilibrium is reached and can be defined by
, an equilibrium is reached and can be defined by![]() ,
, ![]() , and
, and![]() .
.
Theorem 3.4. The system in (22) has a unique endemic equilibrium point if the threshold quantity ![]() and a disease free equilibrium otherwise.
and a disease free equilibrium otherwise.
Proof. When the equilibrium is attained, the right hand side of system (22) goes to zero. Using the third equation in (22), we obtain:
![]() (28)
(28)
Substituting (28) in the second equation of (22) while incorporating (23) gives:
![]() (29)
(29)
where
![]() (30)
(30)
Equation (29) gives ![]() always.
always.
If![]() , then (29) becomes:
, then (29) becomes:
![]() (31)
(31)
But we know that![]() , thus
, thus ![]() and
and
![]()
If ![]() then
then![]() ; if
; if ![]() then
then![]() . But also,
. But also, ![]() since
since
![]() ,
, ![]() , and
, and ![]() which makes the end points
which makes the end points ![]() and
and ![]() for
for![]() . Thus,
. Thus,
![]() is a decreasing function. In this case,there is a unique root
is a decreasing function. In this case,there is a unique root ![]() which accounts for the endemic equilibrium point when
which accounts for the endemic equilibrium point when ![]() and a disease free equilibrium otherwise. ,
and a disease free equilibrium otherwise. ,
If the system approaches a disease free equilibrium, then ![]() (constant) asymptotically and the total population in Equation (21) change according to:
(constant) asymptotically and the total population in Equation (21) change according to:
![]() (32)
(32)
But in this case,![]() . So, if
. So, if![]() , then
, then ![]() decays asymptotically exponentially,
decays asymptotically exponentially, ![]() remains constant if
remains constant if![]() , and grows asymptotically exponentially if
, and grows asymptotically exponentially if![]() . Thus, since
. Thus, since ![]() asymptotically, then from the third equation in (22),
asymptotically, then from the third equation in (22), ![]() asymptotically and also by (23) and (24),
asymptotically and also by (23) and (24), ![]() asymptotically. Therefore, by 3.1, the disease free equilibrium
asymptotically. Therefore, by 3.1, the disease free equilibrium![]() .
.
If the system approaches the endemic equilibrium 3.2, then ![]() asymptotically with
asymptotically with
![]() . From (21), we have:
. From (21), we have:
![]() (33)
(33)
where ![]() and
and
![]() (34)
(34)
![]() decays asymptotically exponentially if
decays asymptotically exponentially if![]() , remains constant if
, remains constant if![]() , and grows asymptotically exponentially if c > 0. Since
, and grows asymptotically exponentially if c > 0. Since![]() , then c ranges from
, then c ranges from ![]() when
when ![]() to
to ![]() when
when ![]() (i.e.
(i.e.![]() ).
).
From (34), we obtain![]() . Substituting this in the third equation of (22), we get
. Substituting this in the third equation of (22), we get![]() . By (23),
. By (23), ![]() which gives the endemic equilibrium
which gives the endemic equilibrium![]() .
.
3.2. Stability Analysis of the Equilibria
To analyze the stability of the equilibria, we establish a Jacobian matrix J and employ the Routh-Hurwitz technique to study the local stability of the equilibria. The Jacobian matrix of the system (22) is given by:
![]() (35)
(35)
At the disease free equilibrium![]() , (35) becomes:
, (35) becomes:
![]() (36)
(36)
From (36), ![]() and
and ![]() and
and ![]() are obtained from:
are obtained from:
![]() (37)
(37)
giving the characteristic equation:
![]() (38)
(38)
The roots of (38) gives:
![]()
Clearly, ![]() always, and if
always, and if![]() , then
, then![]() . Under these conditions, the disease free equilibrium is stable. If
. Under these conditions, the disease free equilibrium is stable. If![]() , then either one or both
, then either one or both ![]() and the disease free equilibrium is unstable.
and the disease free equilibrium is unstable.
Linearizing the model around the endemic equilibrium point, ![]() , the following characteristic equation is obtained:
, the following characteristic equation is obtained:
![]() (39)
(39)
where
![]()
![]()
![]()
![]()
with![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() , and
, and![]() . Since all model parameters are positive, then it is clear that
. Since all model parameters are positive, then it is clear that![]() ,
, ![]() ,
, ![]() , and
, and![]() . But also, if
. But also, if ![]() for
for![]() , then,
, then, ![]() ,
, ![]() , and
, and![]() . Under these conditions,
. Under these conditions, ![]() ,
, ![]() , and
, and ![]() with
with ![]() are always positive. By the Routh-Hurwitz criteria, and by the Descartes rule of signs, the characteristic equation in (39) has roots with only negative real parts. Hence, the endemic equilibrium point
are always positive. By the Routh-Hurwitz criteria, and by the Descartes rule of signs, the characteristic equation in (39) has roots with only negative real parts. Hence, the endemic equilibrium point ![]() is stable. If
is stable. If![]() , then
, then![]() ,
, ![]() , and
, and![]() . Therefore, the endemic equilibrium point is unstable.
. Therefore, the endemic equilibrium point is unstable.
4. Numerical Simulations
This section presents numerical simulation results of the models using parameter values described and presented in Section 4.1. We address the question whether it is necessary to incorporate stages of disease progression when modelling the spread of HIV/AIDS and seek to understand the effect of incorporating stages of HIV progression on the overall infection and spread of disease in the population as well as on estimation of future trend. In addition, we compare the two models by studying the effects of varying transmission rates on the models presented in this paper.
4.1. Model Parameterization
In sub-Saharan Africa, the average time lived by individuals is about 50 years. In this case, the natural mortality rate, ![]() is estimated to be 0.02 years−1. In the same region, the average birth rate, b is estimated to be 0.03. Studies have also estimated the waiting times in the first stage of HIV is 2 to 10 weeks while individuals in the asymptomatic stage spend about 10 to 15 years [5] [6] .
is estimated to be 0.02 years−1. In the same region, the average birth rate, b is estimated to be 0.03. Studies have also estimated the waiting times in the first stage of HIV is 2 to 10 weeks while individuals in the asymptomatic stage spend about 10 to 15 years [5] [6] .
On the other hand, the first empirical data in sub-Saharan Africa communities show substantial variations in transmission among stages of HIV infection after sero-conversion [2] [3] . These studies have showed that the rate of HIV transmission within the first two months is about 12 times higher than in the chronic stages. This indicates that transmission rate in the primary stage of HIV progression ![]() is higher than that of individuals in the asymptomatic stage
is higher than that of individuals in the asymptomatic stage![]() .
.
The transmission rate, r as used in the simple model is estimated using the second equation in (3) at the steady state and given as:
![]() (40)
(40)
where ![]() is the endemic equilibrium state of infections which takes any value in the interval
is the endemic equilibrium state of infections which takes any value in the interval![]() . The minimum value that r takes when
. The minimum value that r takes when ![]() is
is ![]() and if
and if ![]() then
then![]() . Therefore,
. Therefore,![]() .
.
From the model with stages, ![]() is a function of
is a function of![]() . From second equation in (22) at equilibrium, and with
. From second equation in (22) at equilibrium, and with ![]() gives:
gives:
![]() (41)
(41)
where ![]() and c is given by Equation (34).
and c is given by Equation (34).
As the rate at which individuals become infected is increased, then ![]() and
and ![]() increases (Figure 1(a) and Figure 1(b)). This indicates that a careful choice of parameters r,
increases (Figure 1(a) and Figure 1(b)). This indicates that a careful choice of parameters r, ![]() and
and ![]() is required in models of HIV transmission. If the rates of infection are at their minimum values, the models show the disease to clear from the population (i.e.
is required in models of HIV transmission. If the rates of infection are at their minimum values, the models show the disease to clear from the population (i.e.![]() ,
,![]() ) and the quantity
) and the quantity ![]() become equal to one. According to the models, a disease persists in the population if the transmission rates are above their minimum values.
become equal to one. According to the models, a disease persists in the population if the transmission rates are above their minimum values.
4.2. The Effect of Stages in HIV Predictions
Figure 2 presents numerical results of the models with and without stages. Because ![]() is as large as y since individuals in
is as large as y since individuals in ![]() progress to
progress to ![]() within a very short period of time, we simulated the case where
within a very short period of time, we simulated the case where ![]() to compare the results of the two models. The results show a clear difference in the prevalence (
to compare the results of the two models. The results show a clear difference in the prevalence (![]() and
and![]() ), and mortality (Figure 2(b) and Figure 2(c)). It is also observed that, prevalence is high for the model with stages and low for the model with a single group of infected individuals.
), and mortality (Figure 2(b) and Figure 2(c)). It is also observed that, prevalence is high for the model with stages and low for the model with a single group of infected individuals. ![]() increases just after the disease starts to exist. It is also observed that the effective threshold values (
increases just after the disease starts to exist. It is also observed that the effective threshold values (![]() ) for the two models are different. For the model with stages,
) for the two models are different. For the model with stages, ![]() and for the model without stages,
and for the model without stages,![]() . The difference in
. The difference in ![]() is contributed by transmission of individuals in the primary stage (Figure 2(a)) as individuals in this stage have a high amount of viruses in the bloodstream, making transmission to others easier [2] [3] [5] [6] .
is contributed by transmission of individuals in the primary stage (Figure 2(a)) as individuals in this stage have a high amount of viruses in the bloodstream, making transmission to others easier [2] [3] [5] [6] .
Because prevalence is high in infected individuals, mortality becomes high (Figure 2(c)). The difference in the mortality curves for the two models is similar to that found in the prevalence curves. The reason for this is that disease related mortality increase is proportional to the prevalence level in the population.
4.3. Effect of Transmission Rates
Figure 3 presents results of infected groups in the two models at different transmission rates. In general, the proportion of individuals in the infected groups increase with increasing the transmission rates. In the simple model, when r is increased from 0.167 to 0.5, ![]() increases from 1.28 to 3.85, and in the staged model, when
increases from 1.28 to 3.85, and in the staged model, when ![]() is increased from 2.0 to 6.0, and
is increased from 2.0 to 6.0, and ![]() from 0.167 to 0.5,
from 0.167 to 0.5, ![]() increases from 1.61 to 4.82. Indicating that the transmission rates affect the results, especially when stages of disease progression are considered.
increases from 1.61 to 4.82. Indicating that the transmission rates affect the results, especially when stages of disease progression are considered.
5. Summary and Conclusion
In this paper, a simple model for HIV transmission has been formulated and extended to incorporate stages of HIV progression. Stability analysis of the models and numerical simulation examples has been performed to understand the impact of stages in estimates. The effect of varying the transmission rates r, ![]() ,
, ![]() and the disease related death rate (
and the disease related death rate (![]() ) has been studied. The results show that the transmission rate is the driving force in the spread of the disease while the disease death rate has a generally little impact. Because of the sensitive effect of the transmission rates, incorporating stages in the model has a profound impact in the model results.
) has been studied. The results show that the transmission rate is the driving force in the spread of the disease while the disease death rate has a generally little impact. Because of the sensitive effect of the transmission rates, incorporating stages in the model has a profound impact in the model results.
Our results indicate that when ![]() and
and ![]() are varied, a switch between the curves in
are varied, a switch between the curves in ![]() occurs at large t. This is an interesting result which needs careful attention when dealing with disease incidence and transmission rate. One can easily draw different conclusions on the relation between the transmission rate and the persistence of new infections. This study has also shown that individuals in the primary stage play a major role in transmitting the disease. If this group can somehow be identified and convinced to refrain from risky behaviours, at least while they are highly infectious, the impact of the epidemic can be reduced.
occurs at large t. This is an interesting result which needs careful attention when dealing with disease incidence and transmission rate. One can easily draw different conclusions on the relation between the transmission rate and the persistence of new infections. This study has also shown that individuals in the primary stage play a major role in transmitting the disease. If this group can somehow be identified and convinced to refrain from risky behaviours, at least while they are highly infectious, the impact of the epidemic can be reduced.
The models produce different results. The model without stages produce estimates that are lower than the HIV estimates produced when stages are included. The nature of curves for y and ![]() is also different. In the model without stages, the curve for the proportion of infected people grows slowly than in the model with stages.
is also different. In the model without stages, the curve for the proportion of infected people grows slowly than in the model with stages.
Although the models formulated are simple based on assumptions, and without fitting them to data, results show the importance of incorporating stages in models of HIV/AIDS. The results can not only be used to study how important stages of HIV infection are in the spread of HIV, but also they are helpful in evaluating the efficiency of HIV/AIDS models used in estimating and projecting the burden of HIV disease.
Acknowledgements
This work was developed from my MSc dissertation submitted to the University of Stellenbosch, South Africa with financial support from the African Institute for Mathematical sciences. The author acknowledges Fritz Hahne for valuable inputs.