1. Introduction
The medical plant “Clammy Inula” (Inula viscosa L.) Aiton (syn. Cupularia viscosa G. et G., Dittrichia viscosa Greuter) (family Compositae), is a perennial weed, native to the Mediterranean. It grows on hill slopes, damp habitats, and roadsides. In folklore medicine, the plant is used for therapeutic purposes, such as diuretic, topical anti-inflammatory, haemostatic and other purposes [1] - [3] . Aqueous extracts of I. viscosa were shown to exhibit antifungal activity in vitro for phytopathogenic fungi [4] [5] . Plant secondary metabolites, such as essential oils and plant extracts were studied for their antimicrobial activities and most essential oils derived from plants were known to possess insecticidal, antifungal, acaricidal, antibacterial and cytotoxic activities [6] [7] . Accordingly, they were intensely screened and applied in the fields of pharmacology, pharmaceutical botany, medicinal and clinical microbiology, phytopathology and food preservation [8] .
The fungus Botrytis cinerea Pers., the causal agent of grey mould, is a serious damaging plant pathogenic fungus of a number of crops worldwide [9] . Chemical control of the disease often depends on frequent fungicide applications. Unfortunately, B. cinerea is a classical “high-risk” pathogen. The occurrence of resistance to different fungicides with specific mode of action, as well as residues of fungicides in food had been frequently reported [10] - [13] . In Palestine, grey mould is one of the most serious diseases of vegetable crops cultivated in greenhouses, and traditionally control is achieved by the application of a number of fungicides including iprodione (Rovral®). Fungicides resistance in B. cinerea against iprodione and a mixture of Carbendazin and Diethfencarb was reported in West Bank of Palestine vegetable greenhouses [14] . Several alternative control practices were developed against B. cinerea to reduce the fungicides impact including medical plant aqueous extracts of old leaves of I. viscose, which inhibited the fungus in vitro, and against the disease on grapes and tomato fruits [15] . In the same direction, Wang et al. [16] [17] reported that leaf extracts of I. viscosa were highly effective in controlling downy mildew of grapevine. Plant extract was shown to be effective not only against grape downy mildew, but also against cucumber downy mildew (Pseudoperonospora cubensis), late blight in potato and tomato (Phytophthora infestans), wheat powdery mildew (Erysiphe graminis) and sunflower rust (Puccinia helianthi).
The objective of this study was to evaluate the antifungal effect of Inula viscose plant extract on the phytopathogenic fungus (Botrytis cinerea) alone, and in combination with a low dose of the fungicide iprodione (Rovral®) in vitro and in vivo.
2. Materials and Methods
2.1. Fungal Isolates, Fungicide, and Plant’s Extract
The B. cinerea isolate (Bc 99) used was obtained from the collection of the Plant Protection Research Center, Faculty of Agriculture, Hebron University, which was isolated earlier from a bean diseased plant. In addition, the well-known B. cinerea isolate Bo5-10 was used well as. The fungicide iprodione (Rovral®, SC 500 g∙l−1 a.i, Rhone Poulenc, France) commonly used against B. cinerea was incorporated in the experimental treatments as well. Concerning plant’s extract, Inula viscose was collected at the flowering stage from different locations in the Hebron area, 40 km South of Jerusalem. Plant samples were air-dried, grinded, and stored in dark colored jars (1000 ml) at 4˚C. Plant’s extract was obtained by adding 400 ml boiling distilled water over 40 g of ground air-dried plant material stored earlier. After ten minutes, the extract was filtered through 0.35 µm sieves and centrifuged at 5000 rpm for five minutes. Stock solution of 10% was autoclaved and stored to be used later.
2.2. In Vitro Assays
Mycelial Growth Rate
The effect of Inula viscose plant extract and the fungicide iprodione on mycelial growth rate of B. cinerea isolates (Bc 99 and Bo5-10) was evaluated in vitro using potatoes dextrose agar (PDA) medium amended with 0.5 g chloramphencol. Flasks, each containing 200 PDA medium were placed on hot plate with magnetic stirrer to dissolve and homogenize the components; flasks were then autoclaved and allowed to cool down to 55˚C - 60˚C. The plant extract stock (10%) and the fungicide stock solutions (1000 μg∙ml−1, a.i) were prepared in distilled water and added to the growth media to give final concentrations of plant extract 0%, 1%, 2%, 3%, and 4%, and the fungicide concentrations of 0.2, 0.5, 1, and 2 μg∙ml−1 a.i. Growth media (14 ml) were dispensed into each Petri plate (90 mm diameter). The experimental design was completely randomized (CRD) with five Petri plates (replicates) for each plant extract and fungicide concentration for each B. cinerea isolate. Amended Petri plates were then inoculated with 5 mm mycelium disks from 5-day-old cultures of the B. cinerea isolates. Plates were then incubated in growth chamber at 22˚C. Colony diameters were measured after 24 and 72 h and the mycelium growth rate (MGR, cm2∙day−1) was calculated using the following equation [18] :
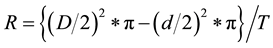
where R―mycelium growth rate, D―average diameter of colony (cm) after 72 h, d―average diameter of colony (cm) after 24 h, π―3.14, and T―time of incubation (day).
The experiment was repeated as described above and the means of MGR of fungal isolates of both experiments are presented.
2.3. Germination Assays
The conidial germination of B. cinerea isolates (Bc 99 and Bo5-10) was evaluated in 24 wells microtiter sterilized plates (Greiner bio-one, Germany). Conidia were harvested from 10 days old B. cinerea cultures growing on PDA medium and incubated at 22˚C, by scraping the mycelium with a glass rod using 10 ml sterile distilled water (SDW). Harvested conidia were filtered using double layers of sterilized muslin membrane to remove all traces of mycelia and placed into sterile tubes. Conidial suspensions were then centrifuged at 2308 ×g (RCF) for 3 minutes and precipitated pellets were washed three times by 10 ml SDW. Conidial concentration of each B. cinerea isolate was then adjusted using the haemocytometer to 2 × 104 conidia∙ml−1. Conidial germination was evaluated in plant extracts at the concentrations (0%, 1%, 2%, 3%, and 4%) and the fungicide iprodione at the concentrations 0, 0.5, 1, 1.5, 2, 3, 4, 5, 7, and 9 μg∙ml−1 (a.i) amended with 1mM glucose. The experimental design was completely randomized using four wells (replicates) of a 24 wells microtiter sterilized plates for each plant extract and fungicide concentration for each B. cinerea isolate. In each well, 480 μl of each plant extract and fungicide concentration and 20 μl of conidial suspension of each B. cinerea isolate were added. Plates were then incubated for 24 hours in growth chamber at 22˚C. Germination in wells was assayed under inverted microscope (Olympus CKX41, Japan). Percentage of germinated conidia was determined by reading five microscope fields and four wells per treatment. The experiment was repeated again as described above.
2.4. In Vivo Assays
Five 40-day-old bean plants (Phaseolus vulgaris) were sprayed (40 ml∙plant−1) until runoff with 0%, 1%, 2%, 3%, and 4% of plant extract and 0, 100, 200, 300, 400, 500 and 600 μg∙ml−1 (a.i) of iprodione against each of the B. cinerea isolates (Bc 99 and Bo5-10). After 3 hours, plants were then sprayed with 20 ml of conidial suspension (2 × 105 CFU) in deionized sterile water containing 2 g∙l−1 glucose and 1 g∙l−1 potassium dihydrogen phosphate (KH2PO4). Inoculated plants were then covered with transparent plastic bags, and incubated in a growth chamber at 22˚C, with a photoperiod of 12 h. Gray mould disease severity was evaluated by estimation of the percentage of leaf mould coverage after 10 days of incubation. The experimental design was completely randomized, where five bean plants were considered as a replicate for each plant extract and fungicide concentration and fungal isolate. The experiment was repeated and from the means of both trials, linear regression was calculated between the disease severity and each concentration of plant extract and fungicide. The effective concentration needed to reduce 50% of the disease (EC50) induced by both B. cinerea isolates was calculated.
2.5. Integrated Control Study
Ten 40-day-old bean plants were grown in 15 cm diameter pots in greenhouse sprayed (40 ml∙plant−1) until runoff with 3.2% of plant extract alone and in another treatment in combination with reduce a dose (330 µg/ml) of iprodione which was measured as EC50. Another treatment involved the use of iprodione alone (330 µg/ml), and finally the control treatment involved spraying plants with SDW. After 3 hours, plants were sprayed again with 20 ml of B. cinerea isolates conidial suspension (2 × 105 CFU) in deionized sterile water containing 2 g∙l−1 glucose and 1 g∙l−1 KH2PO4. Inoculated plants were then covered with transparent plastic bags, and incubated in a growth chamber at 22˚C, with a photoperiod of 12 h. Gray mould disease severity was evaluated by estimation of the percentage of leaf mould coverage after 10 days of incubation. The experimental design was completely randomized, where ten bean plants were considered as a replicate for each treatment and the experiment was repeated.
2.6. Statistical Analysis
The data of mycelial growth rate, conidial germination, and gray mould disease severity were statistically analyzed by using one way ANOVA repeated measurement and Fisher LSD test were used for means separation. (statistical software Sigma Stat® 2.0 program, SPSS Inc., USA).
3. Results
The plant extract at concentration 1% - 4% and the fungicide iprodione at the concentrations (0.2 - 2 µg∙ml−1) significantly reduced the mycelium growth rate (MGR) of B. cinerea both isolates grown on PDA amended medium compared to the control (Figure 1(a) and Figure 1(b)). MGR reduction was positively correlated with increasing Inula viscose extract and iprodione concentrations; MGR was completely inhibited at the highest concentrations tested (4% of I. viscose and 2 µg∙ml−1 of iprodione). Furthermore, it is worth mentioning that I. viscose extract performed well at the lowest concentration tested (1%) and reduced mycelial growth rate by 88% for both isolates, compared to the control.
The variation in MGR between the isolates was not significant. I. viscose plant extract at the concentrations 2% - 4% and iprodione at the concentrations (3 - 9 µg∙ml−1) significantly reduced as well conidial germination of both B. cinerea isolates; reduction was positively correlated with increasing concentrations (Figure 2(a) and Figure 2(b)). Germination was completely inhibited by I. viscose extract at the concentration 4% and iprodione at ≥9 µg∙ml−1 for both isolates. However, results showed that germination of conidia was less affected at lower concentrations. I. viscose showed significant inhibitory effect on germination above 2%. Reduction in germination ranged from (78% - 95%) at the concentrations (2% - 4%) compared to the control. In addition, I. viscose extract at the concentrations 2% - 4% and iprodione at the concentrations (300 - 600 µg∙ml−1) were able to reduce significantly gray mould disease severity (%) on bean plants compared to the controls. Disease severity reduction was positively correlated with increasing I. viscose extract (r2 = 0.97) and iprodione concentrations (r2 = 0.93). Effective concentrations (EC50) of I. viscose plant extract and iprodione against grey mould disease severity on bean plants were 3.2% and 330 µg∙ml−1, respectively (Figure 3(a) and Figure 3(b)). A combination of I. viscose extract at (EC50 = 3.2%) and iprodione at (EC50 = 330 µg∙ml−1) was able to reduce disease severity of bean gray mould by 84% compared to the control. I. viscose extract alone, however, reduced disease severity by 46% and 39%, while iprodione alone reduced disease severity by 70% and 76% for B. cinerea (Bo5-10 and Bc 99), respectively, compared to the control (Figure 4). There were no significant differences between B. cinerea isolates, however, under all treatments tested.
![]()
![]() (a) (b)
(a) (b)
Figure 1. Effect of Inula viscose plant extract at the concentrations (0% - 4%) (a) and the fungicide iprodione at the concentrations (0 - 2 µg∙ml−1) (b) on mycelium growth rate (cm2∙day−1) of B. cinerea isolates (Bc 99 and Bo5-10) (LSD = 0.81).
![]()
![]() (a) (b)
(a) (b)
Figure 2. Effect of Inula viscose plant extract at the concentrations (0% - 4%) (a) and the fungicide iprodione at the concentration (0 - 2 µg∙ml−1) (b) on conidial germination (%) of B. cinerea isolates Bc 99 and Bo5.10 (LSD = 18.8, P ≤ 0.05).
![]()
![]() (a) (b)
(a) (b)
Figure 3. Effect of Inula viscose plant extract (a) and the fungicide iprodione on bean’s gray mould disease severity induced by B. cinerea isolates (Bc 99 and Bo5-10) (LSD = 12.2, P ≤ 0.05).
4. Discussion
The native medicinal plant “Clammy Inula” (I. viscose) showed high antifungal activity in vitro against B. cinerea by reducing the mycelial growth rate and conidial germination; the reduction of MGR and conidial germination was positively correlated with increasing the plant extract concentration. Similar results were observed by several investigators where medicinal plants extracts or oils highly reduced the growth of B. cinerea in vitro; the essential oils of T. vulgaris at the highest concentration (200 ppm) inhibited the MGR by 90.5 % [19] . In the same direction, oil extracts from thyme (C. capitatus) [20] , and sage (S. officinalis) [21] inhibited in vitro mycelial growth of B. cinerea. Furthermore, Boyarz, and Ozcan [22] , showed that the plant extract of Satureja hortensis completely inhibited the mycelial growth of B. cinerea.
Conidial germination of B. cinerea isolates was significantly reduced as well by I. viscosa extract very comparable to the reduction induced by the fungicide iprodione. However, it was obvious that germination of con-
![]()
Figure 4. Effect of Inula viscose plant extract (IV), the fungicide iprodione (IP) and a combination of I. viscose plant extract and the fungicide iprodione (IV + IP) on bean’s gray mould disease severity induced by B. cinerea isolates Bc 99 and Bo5-10 (LSD = 14.8, P ≤ 0.05).
idia was less affected at lower concentrations (e.g. ≤1%) than mycelial growth of the fungus. Similar results were observed by Antonov, et al. [23] , where conidial germination was highly (up to 100%) reduced by the oil extract of several plants. In the same direction, Wilson et al. [24] reported that 13 plant extracts and 4 essential oils inhibited completely the conidial germination of B. cinerea. Furthermore, Ziv [15] showed that aqueous extracts obtained by boiling of old leaves of I. viscosa were inhibitory to fungi in vitro and against B. cinerea on fruits of grapes and tomato.
The water extracts of I. viscosa showed as well strong antifungal properties against B. cinerea in the in vivo bioassays. When plant’s extract were combined with a reduced dose of iprodione, significant reduction (84%) in disease severity was observed. The application of I. viscosa extract alone at the concentration (3.2%), was able to reduce disease severity on bean plants by 46% and 39% induced by B. cinerea isolates (Bo5-10 and Bc 99), respectively. Iprodione at EC50 reduced the disease by 70% and 76% for both isolates, respectively. Similar results were observed by Wang et al., [16] , who showed that leaf extracts of I. viscosa were highly effective in controlling downy mildew of grapevine. In the same direction and in another study, Wang et al., [17] , showed that extracts made from leaves of I. viscosa possess broad-spectrum activity against foliar diseases of several crop plants. Extensive studies had been conducted however, to elucidate the nature or biological activity of plant’s water extracts, essential oils, and whole extracts in organic solvents. Many studies disclosed the presence of phenolics, flavonoids, terpenoids, sesquiterpene acids, sesquiterpene lactones, and other compounds in I. viscosa leaves [25] - [27] .
5. Conclusion
The application of water extracts of I. viscose in combination of a reduced dose of an effective fungicide (e.g. Iprodione) can be a feasible tool in reducing gray mould disease severity. However, further studies are still needed to prove its efficiency in the field under various cropping systems and at a wide scale of application.
NOTES
*Corresponding author.