An Anionic Polymer Incorporating Low Amounts of Hydrophobic Residues Is a Multifunctional Surfactant. Part 1: Emulsifying, Thickening,Moisture-Absorption and Moisture-Retention Abilities of a FattyAcid-Containing Anionic Polysaccharide ()
1. Introduction
We have been studying the function and structure of extracellular polysaccharides (EPS) produced by several Rhodococcus species [1] - [8] , and we demonstrated the potential commercial application of an EPS, prepared from a mucoidal mutant, SM-1, of Rhodococcus rhodochrous ATCC 12674 (SM-1 EPS), as an emulsifier and in moisture-absorbent and moisture-retention materials [1] . SM-1 EPS was found to absorb and retain moisture in both dry and high-temperature environments at a much greater extent than known moisture absorbents or retentioners such as silica gel, glycerol, and hyaluronic acid. SM-1 EPS contains galactose, glucose, fucose, and glucuronic acid at a molar ratio of 6:3:2:4 together with 1.2% (W/W) stearic acid, 2.3% (W/W) palmitic acid, and 10.3% (W/W) pyruvic acid. We also reported the potential for commercial application of several rhodococcal EPSs as thickeners and emulsifiers in addition to its use in bioremediation to clean up oil-spills in marine environments [2] - [4] . These rhodococcal EPSs are high-molecular-weight (>2,000,000) anionic polysaccharides containing fatty acids, mainly stearic acid and palmitic acid, via alkaline-labile bonds, probably ester bonds. We designated these EPS as fatty acid-containing extracellular polysaccharides (FACEPS).
Recently, we determined the sugar-chain structure of one of these rhodococcal FACEPS, the EPS produced by R. rhodochrous strain S-2 (S-2 EPS) [5] . S-2 EPS consists of a tetrasaccharide repeating unit with an → 3)-α- d-mannose-(1 → 3)-α-d-glucose-(1 → 3)-α-d-galactose-(1 → backbone with side chains of β-d-glucuronic acid residues bound to the C-2 position of the mannose residue via glycosyl bond, and it contains 0.8% (W/W) stearic acid and 2.7% (W/W) palmitic acid. However, the relationships between the structure and emulsifying, thickening, moisture-absorption and retention properties of the rhodococcal FACEPS are unclear.
In these two accompanying papers, we address the multifunctional properties of an anionic polymer incorporating a low amount of hydrophobic residues, in particular a fatty acid-containing anionic polysaccharide (this paper, Part 1) and an alkyl-esterified poly-γ-glutamic acid (following paper, Part 2). In this paper, we examined the relationships between the structure and emulsifying, thickening, moisture-absorption and moisture-retention properties of the rhodococcal FACEPS using S-2 EPS, and we suggest that the fatty acid moieties of S-2 EPS underlie these. Furthermore, we also examined the effect of the polysaccharide backbone structure on these properties through the palmitoylation of various polysaccharides that are commercially available.
2. Materials and Methods
2.1. Materials
The S-2 EPS was prepared as described by Urai et al. [5] . Hyaluronic acid from cockscomb was purchased from Tokyo Kasei Kogyo Co. (Tokyo, Japan), and the molecular sieve, glycerol, silica gel, and urea were obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Sumifloc FA-70 (anionic synthetic high-polymer absorbent: polyacrylamide derivative; MW 350 × 104) was purchased from MT Aquapolymer, Inc. (Tokyo, Japan). Alginic acid (Keltone, Kelco Co., Atlanta, GA, USA), carboxymethyl cellulose (Cellogen BS, Dai-Ichi Kogyo Seiyaku Co., Ltd., Kyoto, Japan), and dextran T2000 (Amersham Biosciences Corp., Piscataway, NJ, USA) were also purchased.
2.2. Deacylation of S-2 EPS
Purified S-2 EPS was suspended in 1 M sodium hydroxide aqueous solution and heated at 100˚C for 1 h. After cooling, it was neutralized with aqueous HCl, dialyzed against MilliQ water, and then lyophilized. The resulting deacylated EPS was analyzed by NMR and GC-MS to confirm deacylation.
2.3. Palmitoylation of Polysaccharides
Palmitoylation reactions were conducted as described by Tian et al. with some modifications [9] . Substrates (ca. 200 mg) were soaked in pyridine (2 ml) for 16 h at 80˚C, and then pyridine was removed by drying in vacuo. Dried substrates were immersed in mixtures of 1.7 ml of toluene and 0.2 ml of pyridine and heated at 80˚C for 12 hr. After the mixtures were cooled in an ice-salt bath for 30 min, aliquots (0.12 ml) of palmitoyl chloride (Wako Pure Chemical Industries Co. Ltd., Kyoto, Japan) were slowly added to the reaction solutions with constant stirring. The solutions were cooled in an ice-salt bath for further 30 min, and then heated at 80˚C for 3.5 h. After addition of 50% acetone in MilliQ water (v/v) to each reaction vessel, the reaction solutions were centrifuged at 1000 g for 20 min. Precipitates were washed with 50% acetone in MilliQ water (v/v) again, and then in acetone once. The precipitates were immersed in MilliQ water at 4˚C overnight, and water-soluble palmitoylated polysaccharides was recovered as supernatant by centrifugation at 1000 × g for 20 min. The content of palmitic acid in the palmitoylated polysaccharides was determined by GC-MS after alkali hydrolysis.
2.4. Emulsifying Ability
The polysaccharide was dissolved in 10 ml of MilliQ water with 100 mg of Arabian light crude oil or Louisiana sweet crude oil in a glass vial, and shaken at 110 rpm at 28˚C for16 hour. Emulsification was identified visually.
2.5. Thickening Ability
The viscosity of the native and modified S-2 EPS was determined as described previously [2] .
2.6. Moisture Absorption and Retention Capacities
The moisture absorption and retention capacities were measured as described by Urai et al. [1] . Briefly, constant relative humidity conditions were established at 37˚C. Lyophilized samples were dried at 105˚C overnight, placed in glass weighing vessels and weighed. For determination of the moisture absorption capacity, the samples in the vessels were placed in desiccators for 24 h and then weighed again. The moisture absorption capacity was calculated as follows:
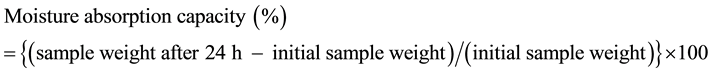
For measurement of the moisture retention capacity, the vessels containing the dried samples were weighed again after addition of MilliQ water (20% of sample weight) with soaking. The vessels were left for 24 h in the desiccators and then weighed. The moisture retention capacity was calculated as follows:
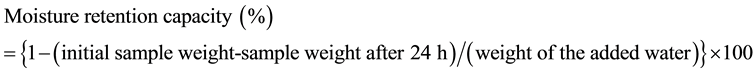
3. Results and Discussion
3.1. Chemical Modification of S-2 EPS
The native S-2 EPS contains 0.8% (W/W) stearic acid and 2.7% (W/W) palmitic acid as described previously [5] . To elucidate the functions of the fatty acid residues of S-2 EPS, the residues were removed by mild alkali hydrolysis (deacylated S-2 EPS, DeAcyl S-2 EPS), and the deacylated S-2 EPS was reacylated by palmitoylation (ReAcyl S-2 EPS). The S-2 EPS was successfully deacylated by mild alkali hydrolysis, as fatty acids were not detected in DeAcyl S-2 EPS by GC-MS and NMR analysis. Palmitoylation was then performed for this DeAcyl S-2 EPS. The palmitic acid content of the ReAcyl S-2 EPS was 1.7% (W/W). DeAcyl S-2 EPS was successfully reacylated with similar amounts of fatty acid to the native S-2 EPS.
3.2. Effects of Fatty Acid Residues on the Emulsifying Ability of S-2 EPS
The emulsifying ability of native S-2 EPS and chemically modified S-2 EPSs was determined visually after shaking with crude oil. The native S-2 EPS showed good emulsifying ability for crude oil in MilliQ water (Figure 1). This ability was completely lost after deacylation, and recovered in ReAcyl S-2 EPS. These results suggest that the fatty acid moieties of S-2 EPS are important for its emulsifying property.
3.3. Effects of Fatty Acid Residues on the Thickening Ability of S-2 EPS
The viscosity of the native S-2 EPS was 4.68 dl/g at 27˚C, which was higher than the 3.90 dl/g for carboxymethyl cellulose (CM-cellulose), suggesting the possibility of its application as a thickener. In contrast, the viscosity of the deacylated S-2EPS was attenuated to 2.73 dl/g, which suggests that the fatty acid moieties of S-2 EPS are involved in its thickening property.
3.4. Characterization of Moisture Retention and Absorption Capacities of S-2 EPS
S-2 EPS retained 79% of its initial moisture (which was equivalent to 16% of the weight of EPS) after 24 h under dry (11% relative humidity, RH) and high-temperature (37˚C) conditions (Table 1). Its moisture retention capacity was much higher than that of commercially available moisture retainers such as hyaluronic acid or glycerol, and slightly higher than that of SM-1 EPS. Under 32% RH, the S-2 EPS showed increased weight, suggesting that the EPS not only retains its initial moisture but also absorbs moisture from dry and high-temperature atmospheres. The S-2 EPS absorbed more than 19% and 8% of its initial weight in moisture under dry (32% and 11%/RH, respectively) and high-temperature (37˚C) conditions (Table 2). This absorption capability was much lower than that of SM-1 EPS but similar to that of other moisture absorbents tested, such as silica gel, glycerol, and hyaluronic acid. These data indicate that S-2 EPS possesses favorable moisture retention and moisture absorption properties.
![]()
Table 1. Moisture retention capacity of S-2 EPS and palmitoylated polysaccharides.
aValues were calculated according to the equation described in the Materials and Methods; bThe weights of samples were greater than their initial weights; cNT, not tested; dThese data are from a previous paper [1] .
![]()
Figure 1. Effect of fatty acid residues on the emulsifying property of S-2 EPS. The final concentration of the EPSs was 0.1 mg/ml, and that of Arabian light crude oil was 10 mg/ml.
![]()
Table 2. Moisture absorption capacity of S-2 EPS and palmitoylated polysaccharides.
aValues were calculated according to the equation described in the Materials and Methods; bNT, not tested; cThese data are from a previous paper [1] .
3.5. Effects of Fatty Acid Residues on the Moisture Retention and Moisture Absorption Capacities of S-2 EPS
DeAcyl S-2 EPS retained far less moisture than the native S-2 EPS (Table 1), and ReAcyl S-2 EPS showed recovery of the moisture retention property, and even under dry (11% RH) and high-temperature (37˚C) conditions, the ReAcyl S-2 EPS showed increased weight, indicating that the EPS not only retains its initial moisture but also absorbs moisture from dry and high-temperature atmospheres. Similarly, the moisture absorption capacity of S-2 EPS was decreased by deacylation, and that of the DeAcyl S-2 EPS was restored or improved by palmitoylation (Table 2). These data suggest that the fatty acid moieties of S-2 EPS play important roles in the moisture retention and moisture absorption properties of the EPS.
Several reports have indicated moisture-retention or moisture-absorption abilities for some polysaccharides. An acidic polysaccharide containing fucose, glucose, rhamnose, and glucuronic acid produced by Alcaligenes latus B-16 is commercially available as a bioabsorbant [10] [11] . Low et al. showed that the starter strain Streptococcus thermophilus MR-1C produces a capsular polysaccharide responsible for the water-binding properties of MR-1C in cheese, and the S. thermophilus MR-1C EPS has a novel basic repeating unit composed of d-galactose, l-rhamnose, and l-fucose in a ratio of 5:2:1 [12] . These polysaccharides possess hydrophobic groups such as methyl glycose in their chemical structures. Hyaluronic acid is widely used in cosmetics as a humectant, and it is an acidic polysaccharide containing acetyl moieties as hydrophobic groups. Lingyum et al. reported that the moisture absorption and moisture retention abilities of carboxymethyl chitosan (CM-chitosan) are dependent on the degree of acetylation [13] . These polysaccharides typically possess hydrophobic groups in their structure, but their polysaccharide backbone structure varies. We therefore examined the effect of the polysaccharide backbone structure on the emulsifying, moisture retention, and moisture absorption properties by palmitoylating various commercially available polysaccharides.
3.6. Effect of Palmitoylation on Emulsifying, Moisture Retention, and Moisture Absorption Properties of Commercially Available Polysaccharides
CM-cellulose, alginic acid, and dextran were palmitoylated, and the palmitic acid content was determined by GC-MS as 0.3%, 0.9%, and 0.2% (W/W), respectively. Figure 2 shows the emulsifying property of the palmitoylated polysaccharides. Palmitoylated alginic acid and CM-cellulose showed effective emulsification of crude oil, but palmitoylated dextran did not. These polysaccharides could not emulsify crude oil before palmitoylation (data not shown). Because alginic acid and CM-cellulose are acidic and dextran is a neutral polysaccharide, the acidic behavior of the polysaccharide backbone is important to for the emulsification mediated by palmitoylation.
The moisture retention and absorption properties of palmitoylated polysaccharides were also tested (Table 1 and Table 2, respectively). After palmitoylation, these properties were improved by approximately 1.7 fold in CM-cellulose, whereas the other polysaccharides showed a reduction rather than an improvement. These data suggest that the chemical structure of the sugar chain also affects the moisture retention and moisture absorption properties of the polysaccharide. The moisture retention and absorption properties of the Deacyl S-2 EPS and CM-cellulose, but not those of alginic acid and dextran, were improved by palmitoylation. Deacyl S-2 EPS and CM-cellulose share structural similarity with regard to acidity. Deacyl S-2 EPS contains one carboxyl group for every four glycose residues, and the CM-cellulose used in this study contains one carboxyl group for every two glucose residues. In contrast, alginic acid is a highly acidic polymer that contains a carboxyl group for every glycose residue. Dextran is a neutral homopolysaccharide consisting of glucose. Lingyum et al. described that
1 2 3 4 5
![]()
Figure 2. Effect of palmitoylation on the emulsifying property of commercially available polysaccharides. The final concentration of the polysaccharide was 0.5 mg/ml, and that of Louisiana sweet crude oil was 10 mg/ml. 1, No addition of polysaccharide; 2, palmitoylated alginic acid; 3, palmitoylated CM-cellulose; 4, palmitoylated dextran; and 5, S-2 EPS.
the degree of carboxymethylation affects the moisture absorption and moisture retention abilities of the CM- chitosan [13] , and they suggested that carboxyl groups are important for the ability of CM-chitosan to absorb and retain moisture. However, a high degree of carboxymethylation causes strong hydrogen bonding between CM-chitosan chains, which prevents the adsorption and retention of moisture. In the case of the palmitoylated polysaccharide, the acidity of the polysaccharide backbone may be similarly involved in the moisture absorption and retention properties.
4. Conclusion
We showed that the fatty acid moiety of the rhodococcal FACEPS may be involved in the emulsifying, thickening, moisture-absorption and moisture-retention properties of the FACEPS. We also showed that the acidity of the polysaccharide backbone may play a role in these properties. To our knowledge, this is the first report of the multifunctional properties of an anionic polymer incorporating a low amount of hydrophobic residues. These observations are useful to create new multifunctional surfactants from renewable raw material with the potential to be used in various industries, e.g., cosmetics. To determine how the hydrophobic groups incorporated into the anionic polymer contribute to the high capacity of emulsification, moisture absorption and moisture retention, it is necessary to synthesize a polymer having a more simplified structure than polysaccharides. In the following paper (Part 2), we synthesized alkyl-esterified poly-γ-glutamic acid with various lengths of alkyl chains and degrees of substitution to examine the effect of the hydrophobic groups on the emulsifying, moisture absorption, and moisture retention properties of an anionic polymer.
Acknowledgements
We are grateful to T. Beppu for giving us the opportunity to complete this research. We thank K. Ueda at the Institute of Applied Life Science for allowing us to use its instrumental facilities. We also thank T. Fukuhara for his valuable discussion. We acknowledge H. Anzai and various members of our laboratories for their suggestions, encouragement, and technical assistance. This study was supported in part by grants from the Takano Life Science Research Foundation (Japan).
NOTES
*Corresponding author.