Acute Toxicity (Lethal Dose 50 Calculation) of Herbal Drug Somina in Rats and Mice ()
1. Introduction
Somina (Herbal drug) prepared by Hamdard Laboratories (Waqf) Pakistan in powdered form is widely used in Unani system of medicine for the treatment of mental illness. It is claimed that somina possesses sedative, hypnotic and anxiolytic activities [1] . Somina is composed of five different medicinal plants.
Sesamum indicum, Prunus amygdalus, Papaver somniferum, Lactuca serriola (seed extracts), Lagenaria vulgaris.
In the literature, different properties of these medicinal plants have been reported. Sesamum indicum was reported to have antioxidant [2] and anti-inflammatory activities [3] . Prunus amygdalus keeps medicinal properties such as anti-inflammatory, sedative, anti-hyperlipidemia, antitumor and antioxidant and Antimicrobial [4] . Anticonvulsant [5] and analgesic activity [6] of Papaver somniferum were cited in the literature. Lactuca serriola was found to possess spasmogenic, spasmolytic, bronchodilator, and vasorelaxant activities [7] . Since ancient times Lagenaria vulgaris has been utilized for treatment of jaundice, diabetes, ulcer, piles, colitis, hypertension and skin diseases [8] . Despite the popular use of these plants, some toxicological studies have previously performed and the results showed that at different doses Lagenaria vulgaris (2 g/kg: [9] ), Lactuca serriola (6 g/kg; [10] ), Prunus amygdalus (2 g/kg: [11] ), Sesamum indicum (500 mg/kg: [12] ) were found to be well tolerated. However, toxicity or safety of Individual constituents has reported in the literature but screening has not yet done on somina as whole to confirm its safety for the use in folkloric medicine. This study was conducted to investigate the toxicity of somina having all ingredients together.
2. Materials and Methods
2.1. Formation of Different Doses of Somina
Somina is available in powder form. Recommended dose of somina for human is 10 g/70kg. In the present study, different doses of somina were used as shown in Table 1.
All doses were prepared by dissolving its powder in distilled water at the time of administration for the determination of LD50.
2.2. Animals
40 Adult NMRI mice (20 - 25 g) and 40 adult Sprague-Dawley Rats (200 - 250 g) of either sex were obtained from Dr. Hafiz Muhammad Ilyas Institute of Pharmacology and Herbal Sciences (Dr. HMIIPHS) and were housed in groups of 5 per cage for seven days prior to experimentation in an ideal laboratory environment as per OCED [13] . Each experimental group consisted of five animals. University and Departmental committee for Research and Ethics had approved all the experimental protocols. Each animal was used only once. For ethical reason, all animals were sacrificed at the end of the study [14] .
2.3. Toxicological/Safety Evaluation Studies in Mice
Eight groups containing five NMR-I mice (25 - 30 g) in each and eight groups containing five rats (200 - 250 g) were used in this study. All animals were treated orally once and different doses (control, 10, 100, 285, 500, 1000, 5000, 10,000 mg/kg) were administered as shown in Table 1.
Animals were weighed before the dose administration. All the animals were kept under continuous observation for 6 hours after the administration of the dose, for any change in behavior or physical activities. After 24 hrs, all survived mice were anesthetized with pentothal sodium (40 mg/kg) and autopsied.
![]()
Table 1. Different doses of somina (herbal drug).
2.4. Calculation of Median Lethal Dose (LD50)
For each mouse, the observation was made for 24 hr and symptoms of toxicity and rate of mortality in each group were noted. At the end of study period, expired animals were counted for the calculation of LD50. The arithmetic method of Karber [15] was used for the determination of LD50.
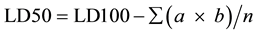
n = total number of animal in a group.
a = the difference between two successive doses of administered extract/substance.
b = the average number of dead animals in two successive doses.
LD100 = Lethal dose causing the 100% death of all test animals.
Hodge and Sterner scale (Table 2) was used for the evaluation of toxicity with the help of LD50 [16] .
3. Results
From the experiment, the results reveal that the somina has not been found to be toxic even at 10,000 mg/kg or 10 mg/kg that is 70 times higher than the human dose in experimental animals (rats and mice) as shown in Table 3. The animals received 10,000 mg/kg orally was not found to cause any mortality and non-significant changes were observed in wellness parameters used for evaluation of toxicity. Behavioral pattern like salivation, sleep cycle and corner sitting of the treated animals were found to enhance (Table 3). However, the low doses did not produce any pronounced effect. Autopsy revealed that no changes were observed in organ structure and weight.
![]()
Table 2. Hodge and sterner toxicity scale.
![]()
Table 3. Toxicological study of different doses of somina administered orally in mice and rats.
LD50 Value: As per observations and calculations (Karber, 1931), the LD50 value of somina after oral administration was found to be more than 10,000 mg/kg body weight.
According to Hodge and Sterner (2005) toxicity scale, somina is said to be in non-toxic herbal drug category (Table 2).
4. Discussion
Although, the somina is used in folkloric medicine for the treatment of mental illness and research had been done to investigate its said effect [1] [17] but the present study was conducted to reveals the safety evaluation of somina, because it contain different constituent and it is a mixture of five medicinal plants. However, each plant contains different active compounds have medicinal and toxic effects. That’s why it is mandatory to evaluate the toxicity of herbal drugs (somina) whose adverse effects and toxic doses are mostly unknown [18] . Previously reported data revealed the toxic effect of different herbs at different doses such as Kava, germander (Teucriumchamaedrys). Chaparral (Larrea tridentate) causes severe liver injury [19] . Licorice can induce hypokalemic myopathy [20] and Kelp (seaweed) can cause hyperthyroidism [21] . In the present study, the somina was found to be safe up to 10,000 mg/kg orally.
This present study is in agreement with other previous studies in which different doses of constituent of somina is reported to be safe like Lagenaria vulgaris at the dose of 2 g/kg [9] , Lactuca serriola at the dose of 6 g/kg [10] , Prunus amygdalus at the dose of 2 g/kg [11] , while Sesamum indicum did not produce any toxicity up to the dose of 500 mg/kg [12] . In the present study, a maximum dose of somina was used in all above of reported literature doses were less than the present study. Although the present study confirms that somina is practically non-toxic [16] . In the present study, other organs like kidney, heart and spleen did not show any significant change.
5. Conclusion
In conclusion, the results of the present study conclude that somina is safe or practically non-toxic when administered orally. This study is the preliminary study; in the future, this research is offering an outset to continue the research by administering the somina through different routes in different animals’ species and in human.
Acknowledgements
Author is grateful to Prof. S. I Ahmed (Late) Former Dean Faculty of Pharmacy, University of Karachi, Hamdard University Karachi and Director of HMIIPHS, Hamdard University Karachi for his support, encouragement at every step of this study and Hamdard Foundation Pakistan for financial support.
Ethical Approval
Author hereby declared that the experimental protocol was approved by the University and Departmental committee for Research and Ethics. Each animal was used only once. For ethical reason, all animals were sacrificed at the end of the study (AVMA Guideline, 2013). Experimental protocol was followed according to Guidelines for Care and Use of Laboratory Animals in Biomedical Research (2010). All rules were followed as well as specific national laws where applicable.
Competing Interests
The author has no conflict of interest to report.