Waste Water Treatment in Direct Borohydride Fuel Cell with Bipolar Membrane ()
1. Introduction
Water is the unique resource that cannot be substituted by any alternative and it should be managed and conserved. Unfortunately, due to the intensive use of water for industrial purposes, sharply deteriorating water quality and ecosystem balance are infringed. Polluted water is one of the main causes of diseases of live organisms, often with lethal end.
Various technologies offered for water treatment, such as gravitational settling, filtration, aerial separation or adsorption over activated carbon are mechanical processes accompanied by streams of wastes after treatment, often containing toxic, biologically non-degradable organic substances. Advanced oxidation processes (AOPs) have been offered to resolve these problems. One of the most frequently used AOPs is Fenton reaction, based on hydroxyl radical formation at H2O2 decomposition in the presence of Fe2+ ions in acidic medium [1] -[12] :
 (1)
(1)
Fenton reaction is performed in acidic medium (pH ~ 3) for keeping Fe2+ catalyst in soluble form and for increasing of hydroxyl radical (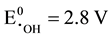 ) oxidation intensity. In the process of obtaining
) oxidation intensity. In the process of obtaining  radicals, we can avoid abundant Fe3+ accumulation (that slows down treatment process) by reduction photolysis [Fe(OH)]2+ leading to Fe2+ ions regeneration with additional yield of
radicals, we can avoid abundant Fe3+ accumulation (that slows down treatment process) by reduction photolysis [Fe(OH)]2+ leading to Fe2+ ions regeneration with additional yield of 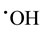 [13] -[15] :
[13] -[15] :
 (2)
(2)
In addition, UV radiation can induce the photodegradation of some oxidation by-products or their complexes with Fe3+ promoting Fe2+ regeneration. This is the case of the photodecarboxylation of Fe3+ carboxylate species according to the total reaction [15] :
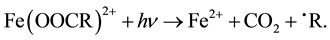 (3)
(3)
Hydrogen peroxide is one of the most important chemical reagents and it is attributed to a “green reagents” group, since in red-ox reactions, it decomposes into environmentally friendly products―water and oxygen. Industrial hydrogen peroxide manufacturing enterprises are built only at several sites and its manufacturing is based on so-called “antraquinone process”, which needs centralized huge hydrogen production plants for the feedback. Delivery, storage and handling of hazardous concentrated solutions of the strong oxidant are costly and risky. Therefore, development of methods for on-site generation of H2O2, especially at water treatment, is an important issue.
The main goal of the present paper is development of electrochemical method for H2O2 generation through cathode reduction of oxygen and decontamination of water from organic pollutants in fuel cell systems.
Hydrogen peroxide electric generation in acidic solutions (pH 3) through two-electron oxygen reduction over gas-diffusion carbon electrode was realized by the authors
 (4)
(4)
“in-cell” and “ex-cell” for on-site water treatment, contaminated with phenol, 4-chlorophenol, aniline and other biologically non-degradable organic pollutants, by electro-Fenton reaction [16] -[18] .
In all systems of fuel cells with hydrogen peroxide cogeneration, given in references, oxygen reduction is realized in neutral or alkali media in cells, divided by cathion- or anion-exchange membranes, according to the reaction [19] -[25] :
 (5)
(5)
Performance of AOPs in these systems with generated Н2О2 is available only at constant correction of ca- tholyte in acid media. Application of bipolar membrane in fuel cell enabled us to keep constant рН 2.5 - 3.3 in the catholyte. Bipolar membrane consists of cathionite and anionite layers, which are in intimate contact. The cathion selective side of the membrane is directed towards the cathode and the anion-selective side is directed towards the anode. Water from the anolyte and catholyte is diffused towards the junction of two layers of the bipolar membrane, where water at the impact of electric field is dissociated into Н+ and ОН− ions. The obtained Н+ ions migrate towards the cathode through the cathion-exchange layer and ОН− ions towards the anode via the anion-exchange layer of the membrane. As a result, cations and anions deficit in the catholyte and anolyte is compensated at the expense of corresponding ions, contributing to keeping of initial pH 2.8 - 3.2 in a catholyte [26] .
We have developed original direct borohydride fuel cell (DBFC) with bipolar membrane that generates hydrogen peroxide (Figure 1) for treatment of water contaminated by phenol and pesticides―valsarel, valsaciper and BI-58. Reactions at the electrodes:
![]()
Figure 1. Schematic representation of ion migration in DBFC with bipolar membrane generating hydrogen peroxide.
anode: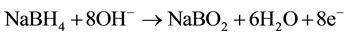
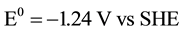 ;
;
cathode: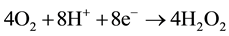
 ;
;
total:
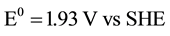 .
.
Reaction behavior in bipolar membrane at the junction of two oppositely charged layers:
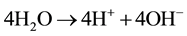
Potential, equal to 0.83 V, is necessary for water dissociation in bipolar membranes [26] . DBFC electromotive potential equals to E0 = 1.93 - 0.83 = 1.1 V which is quite sufficient for the fuel cell operation.
2. Materials and Methods
Solutions were prepared by dissolution of chemical grade reagents, potassium chloride and sodium hydroxide in distilled water. Phenol and commercially accessible pesticides: BI-58 (400 g/LC5H12NO3PS2―dymethoate), val- saciper (250 g/L cypermethrin-α-cyano-3-phenoxybenzyl2,2-dichlorvinyl-2,2-dimethyl-3-(2,2dichlorvinyl) cy- clopropane carboxylate) and valsarel (48% chlorpyrifos, 5% cypermethrin) were used as organic pollutants of water.
Electrode made of carbonaceous fiber covered by non-catalyzed carbon black layer “BlackPearls 2000” (ElectroCellAB) was used as cathode. Electrode 40% Pt 0.5 mg/cm2 with XC 72R carbonaceous black on Carbon Cloth was used as an anode. Anode and cathode compartments of DBFC were separated with bipolar membrane Fumasep® FBM (Fumatech Inc.). UV irradiation was performed by the use of quartz construction “tube in tube”, that was placed in circulation contour of a catholyte. UV lamp with wave length 253 nm (8 W) was placed into inner tube, catholyte circulated between tubes. Current, voltage and pH were measured by M2015 ammeter, M106 high-resistance voltmeter and HI 8424 microcomputer pH-meter, correspondingly.
The concentration of ![]() accumulated in the electrolyte was determined by standard titration method with KMnO4 [27] . Presence of Fe2+ and Fe3+ in catholyte was controlled by qualitative reaction by the use of potassium ferricyanide and potassium ferrocyanide, correspondingly. Chemical oxygen demand (COD) was determined by spectrophotometer DR-4000 UV (see suitable instruction).
accumulated in the electrolyte was determined by standard titration method with KMnO4 [27] . Presence of Fe2+ and Fe3+ in catholyte was controlled by qualitative reaction by the use of potassium ferricyanide and potassium ferrocyanide, correspondingly. Chemical oxygen demand (COD) was determined by spectrophotometer DR-4000 UV (see suitable instruction).
Principal scheme of DBFC is offered in the Figure 2, where 1 and 2 are cathode and anode compartments made of plexiglass, each of the size (10 × 10) сm; 3―cathodic and anodic spacers, places among rubber gaskets 4; oxygen (or air) is fed from compressor via gas chamber 5 to gas-diffusion cathode 6 posed on nickel mesh 7; anolyte is fed to anode 9 through nickel mesh feeder 10; cathode and anode compartments are separated by bipolar membrane 11 [25] .
The experimental setup is shown in the Figure 3. Anolyte and catolyte were circulated through the anode and cathode compartments of the DBFC to the corresponding tanks by means of pumps. Pressure of air fed from the air-pump to the gas chamber was controlled by manometer. The external load was changed using a box of resistors (R), and the FC parameters were controlled by an ammeter and a high impedance voltmeter [28] .
![]()
Figure 3. Experimental plant DBFC for treatment of model solution.
3. Results and Discussion
3.1. Effect of Cell Operation Length on Generated Current Density, Hydrogen Peroxide Rate and Catholyte pH
For Fenton reaction catholyte pH should be kept within the limits of 2.5 - 3.2. We used fuel cell divided by bipolar membrane to keep pH in these limits. Anode and cathode were 9 cm2 area; catholyte―0.5 M NaCl, while anolyte―2 M NaOH + 2 M NaBH4, air pressure in the air chamber―300 Pa, circulation rate of the electrolytes was 1000 ml/min. After 60 min operation of DBFC current density equaled to 10 mA/cm2, catholyte pH 2.6 and practically was not changed further, by time (Figure 4).
In DBFC generated hydrogen peroxide current efficiency in time is offered in the Figure 5. In one hour it was changed from 100% to 78%, which is probably associated with H2O2 autocatalytic decomposition because of increase of its concentration in gas-diffusion electrode pores.
3.2. Electro-Fenton Process in Fuel Cell
Electro-Fenton process was performed in cathode compartment of the DBFC. 500 ml catholyte circulated at the support of pump at 1000 ml/min rate.
![]()
Figure 4. Variation of the DBFC generated current density (1) and catholyte pH (2) in time. Catholyte―0.5 M NaCl, anolyte―2 M NaOH + 2 M NaBH4; t = 20˚C, circulation rate of electrolytes―1000 ml/min, air pressure 300 Pa.
![]()
Figure 5. Variation in hydrogen peroxide current efficiency with time. Catholyte―0.5 M NaCl, anolyte―2 M NaOH + 2 M NaBH4, t = 20˚C, circulation rate of electrolytes―1000 ml/min, air pressure 300 Pa.
Phenol (COD = 500 ppm) was introduced in advance into 0.5 M NaCl containing catholyte were 0.1 - 1.0 mM Fe2+ ions (in the form of FeSO4∙7H2O) was added. 2 M NaOH solution circulated in anode compartment (Figure 6). To determine grade of solution decontamination from phenol, samples were taken from circulation tank and chemical oxygen demand (COD) was defined periodically.
In the electro-Fenton process Fe2+ regeneration proceeds according to the reactions 2 and 3, rates of these reactions are rather low [29] , therefore Fe3+ formed as a result of Fenton reaction fails to manage complete reduction and it is accumulated in the solution, contributing to decrease of purification grade from organic substances. In addition, at two-electron oxygen reduction gas-diffusion electrode pores and surface are alkalized and electrode surface is covered by thin layer of Fe3+ hydroxide, which exerts negative effect on the treatment process. That is, at the operation of gas-diffusion cathode, selection of Fe2+ optimal concentration in catholyte is most important.
Figure 7 shows that at the terms of 1 mM Fe2+ ion concentration in catholyte, current that is generated in external circuit of fuel cell decreases that is caused by settling of Fe3+ hydroxides on gas-diffusion cathode surface. Such conditions electro-Fenton process is hindered and thorough mineralization of organic substances does not
![]()
Figure 6. Variation of COD in time at treatment of phenol containing catholyte by Fenton method at various Fe2+ concentrations. Catholyte―0.5 M NaCl + X mM Fe2+, pH 2.6, anolyte―2 M NaOH + 2 M NaBH4, t = 20˚C, circulation rate of electrolytes―1000 ml/min, air pressure 300 Pa.
![]()
Figure 7. Variation of the DBFC generated current in time at various Fe2+ concentration in catholyte. Catholyte―0.5 M NaCl + X mM Fe2+, pH 2.6, anolyte―2 M NaOH + 2 M NaBH4, t = 20˚C, circulation rate of electrolytes―1000 ml/min, air pressure 300 Pa.
take place. At a decrease of Fe2+ concentration in catholyte to 0.5 mM, gas-diffusion electrode surface is less covered by Fe3+ hydroxides and the process of mineralization of organic substances suffers significant improve- ment. But the better result is obtained at 0.1 mM Fe2+ in catholyte. At this moment the greater part of gas-diffu- sion electrode surface is not covered by hydroxides and current generation in the initial three hours decreases from 150 mA to 130 mA and then remains stable for the following two hours.
Thus, proceeding from the received results, optimum concentration of Fe2+ for removal of phenol from water makes 0.1 mM that corresponds to data of other references [29] .
In Figure 8 change in a catholyte of DBFC of COD of pesticides―valsaciper, valsaren and BI-58 in the course of mineralization by the application of electro-Fenton methodis shown.
To improve efficiency of treatment of water contaminated by organic pollutants was used UV irradiation, which enables to regenerate Fe2+ ions (reactions 2 and 3), and provide with an additional source of hydroxyl radicals:
![]() (6)
(6)
![]()
Figure 8. Variation of COD at mineralization of pesticides in time, by the application of electro-Fenton method. Catholyte―0.5 M NaCl + 0.1 mM Fe2+ pH 2.6, anolyte―2 M NaOH + 2 M NaBH4, t = 20˚C, circulation rate of electrolytes―1000 ml/min, air pressure 300 Pa.
3.3. Treatment of Model Water Polluted by Organic Substances in DBFC by the Use of UV Radiation
As is known, at the impact of UV radiation on hydrogen peroxide strong oxidants―hydroxyl radicals (•OH) are obtained and by their application treatment of waters containing various organic substances was performed. To prove the possibility of efficient treatment of waters contaminated by organic pollutants, by means of UV radiation in fuel cell, we used DBFC with bipolar membrane, and studied degradation process of phenol, BI-58, valsarel and valsaciper in model waters. The external circulating contour of a catholyte (500 ml 0.5 M NaCl) represented a “tube in tube” construction. In the inner tube UV radiation lamp was placed. Hydrogen peroxide- containing catholyte circulated in the space between tubes at 1000 ml/min rate. 2 M NaOH + 2 M NaBH4 solution served as anolyte. Current that was generated during 4 hr operation of the fuel cell decreased from 150 mA to 135 mA. To check the process of decomposition of organic substances in catholyte the chemical oxygen demand (COD) was measured in periodically taken samples. Results are given in Figure 9, which shows that at the impact of hydroxyl radicals obtained at the interaction of UV radiation and hydrogen peroxide, the process of degradation of organic pollutants was achieved in the initial 15 - 20 min, but then the process practically stopped. Thus, degradation of organic pollutants by interaction only of hydrogen peroxide with UV radiation was inefficient.
3.4. Treatment of Model Waters Polluted by Organic Substances in DBFC by Photo-Electro-Fenton Method
A 0.1 mM Fe2+ were added to catholyte (500 ml 0.5 M NaCl) that was polluted by phenol, BI-58, valsarel and valsaciper in different tests, and then was treated by photo-electro-Fenton method. Initial COD of catholyte was 500 ppm for each compound. All other terms of tests were analogous to previous ones.
As is seen from Figure 10 application of photo-electro-Fenton process in fuel cell appeared successful: practically complete mineralization of the selected organic substances, less phenol (the mineralization of phenol made 20 ppm), was achieved at 180 min. Fe2+ ions regeneration process slow down, due to decreasing ultra violet rays permeability in dark intermediate products formed at phenol oxidation process, which finally was reflected on the treatment process. In the case of other organic substances the process of Fe2+ generation at the impact of UV rays is permanent and settling of Fe3+ hydroxides at gas-diffusion electrode is not observed, since current that is generated in fuel cell is stable and the process of organic substances degradation progresses more efficiently than at using of electro-Fenton process.
Phenol turned out most stable among organic substances selected by us. Therefore, phenol was given preference in the study of effect of temperature on its process of degradation by photo-electro-Fenton method
![]()
Figure 9. Variation of COD in time at UV radiation of catholyte polluted by organic substances in DBFC. Catholyte―0.5 M NaCl, pH 2.6, anolyte―2 M NaOH + 2 M NaBH4, t = 20˚C, circulation rate of electrolytes―1000 ml/min, air pressure 300 Pa.
![]()
Figure 10. Variation of COD in time at the treatment of catholyte polluted by organic substances by photo-electro-Fenton method. Catholyte―0.5 M NaCl + 0.1 mM Fe2+, pH 2.6, anolyte―2 M NaOH + 2 M NaBH4, t = 20˚C, circulation rate of electrolytes―1000 ml /min, air pressure 300 Pa.
(Figure 11). The results show that at the increase of temperature degradation of phenol sharply increases: at 20˚C after 120 min COD decreases from 500 ppm to 117 ppm, at 40˚C to 15 ppm and at 60˚C to 10 ppm; after 180 min at 40˚C and 60˚C COD suffers practically similar change and falls to 5 ppm. Intensification of phenol degradation at the increase of temperature is conditioned by increase of generated current intensity. Thus, at 20˚C instead of 50 mA a fuel cell generated 200 mA at 40˚C and at 60˚C - 240 mA.
We studied effect of phenol concentration on its degradation process. Therefore phenol containing three concentration model water (500 ml, 0.5 M NaCl) samples were taken with starting COD 500, 300 and 120 ppm (Figure 12). Other terms of experiment were the same. Figure 12 shows that decrease of initial phenol concentration is positively reflected on the process rate. In the case of 500 ppm (start concentration) in 90 min mineralization reached 150 ppm; in the case of 300 ppm in the same time―10 ppm; in the case of 120 ppm―3 ppm.
Decomposition of phenol followed first-order kinetics as described by the following equation:
![]() , h−1 (7)
, h−1 (7)
![]()
Figure 11. Influence of temperature on phenol degradation. Catholyte―0.5 M NaCl + 0.1 mM Fe2+, pH 2.6, anolyte―2 M NaOH + 2 M NaBH4, t = 20˚C, circulation rate of electrolytes―1000 ml/min, air pressure 300 Pa.
![]()
Figure 12. Influence of concentration of phenol on СОD of water in time. Catholyte―0.5 M NaCl + 0.1 mM Fe2+, pH 2.6, anolyte―2 M NaOH + 2 M NaBH4, t = 20˚C, circulation rate of electrolytes―1000 ml/min, air pressure 300 Pa.
where k is the degradation rate constant of phenol; k was determined experimentally by plotting― ln (COD/COD0) versus the reaction time (Figure 13). The reaction rate was determined for the first 30 min of phenol degradation. The value of the pseudo-first order kinetic constant for concentaration 120 ppm of phenol COD is 6.35 h−1 [30] [31] .
4. Conclusion
Filter-press type DBFC divided with bipolar membrane (Fumasep® FBM Fumatech Inc., Germany) was construed. Geometrical area of the gas-diffusion cathode and of the anode was 9 cm2 each. It was established that application of bipolar membrane enabled to keep constant pH in catholyte within 2.5 - 3.2 limits, which allowed us to carry out treatment of water polluted by organic compounds in fuel cell catholyte by electro-Fenton and photo-electro-Fenton methods. Optimal concentration of Fe2+ ions in the applied methods was determined which equaled to 0.1 mM; mineralization of model waters polluted by phenol, valsaren, valsaciper and BI-58 was carried out. With the view of efficiency, photo-electro-Fenton method of treatment was the most efficient, which enabled to decrease COD of catholytes containing (in each case) phenol, valsaren, BI-58 and valsaciper from
![]()
Figure 13. Comparison of the decrease of the phenol COD concentration and Neperian logarithm of the relative COD vs time.
500 ppm to 30, 11, 9 and 3 ppm, respectively after 180 min treatment. At the increase of catholyte temperature from 20˚C to 40˚C in the same period, phenol COD fell to 5 ppm.
Acknowledgements
The designate project has been fulfilled by the support of Shota Rustaveli National Science Foundation. Any idea in this publication is possessed by the authors and may not represent the opinion of Shota Rustaveli National Science Foundation.
NOTES
*Corresponding author.