Influence of Thickness on the Photosensing Properties of Chemically Synthesized Copper Sulfide Thin Films ()
1. Introduction
Semiconductor metal chalcogenide thin films have a potential application in the field of the energy storage, gas, and photo-sensing mechanism [1] -[4] . Their semiconducting nanocrystalline nature together with electronic and optical properties makes them highly suitable for various opto-electronic applications. Among the available metal chalcogenides, copper sulfide (CuS) is an important semiconducting material that has been widely used in the field of solar cells, photocatalysts, supercapacitors, gas sensor, etc. [5] - [8] . In recent years, efforts have been devoted to the formation of the complex structure and to control the valence state in copper based chalcogenide compounds. It is reported that CuS has five stable phases that exists at room temperature (RT). These are covellite (CuS) in the sulphur-rich region, anilite (Cu1.75S), digenite (Cu1.8S), djurleite (Cu1.95S), and chalcocite (Cu2S) in the copper-rich region [5] . Among these, CuS (covellite) exhibits metal-like electrical conductivity and it also possesses near-ideal solar control characteristics. Notwithstanding to this, they even show easy current-conduc- tion and charge-transfer mechanism [9] . During the last decade, preparation of the CuS thin film became commercialized due to its stable opto-electronic performance and was found to be a suitable candidate for the photo- sensitive devices [10] , which could lead to next generation displays [11] as well as touch screens [12] . Moreover, all these applications are based upon the property of electrical conductivity, which is rather one of the significant properties found in CuS.
There are several reports found on synthesis and characterization of CuS thin films deposited by different physical as well as chemical methods. For instance, Yuan et al. [13] reported on thickness dependent CuS thin films and found that with lowering the thickness, the transmittance of CuS thin films became larger. In addition to this, they used these CuS thin films as front contact layers and found a photoelectric response. Bollero et al. [14] also studied the influence of thickness on the optical and electrical properties. Interestingly, they found that the opto-electronic properties of the films did not changed much even after storing the samples for a year under laboratory ambient conditions. Furthermore, Naşcu et al. [15] studied CuS films both in dark and under illumination and found that the electrical resistance values decreased with illumination. In another study done by Yildirim et al. [16] on CuS, they found that the films were photosensitive and the photosensitivity increased with increase in light intensity. Moreover, they even reported that after annealing of these CuS films, the photosensitivity drastically decreased as compared to the as-deposited CuS films.
Although large amount of literature is available on the CuS thin film, but little is found on CuS as photosensors. In addition to this, the influence of thickness on the photo-sensitive CuS thin film is not properly studied and explained. Therefore, considering all the above mentioned facts, here in the present study we report about the influence of the thickness on the physical as well as photo-sensor properties of p-type CuS thin films synthesized by the simple chemical bath deposition (CBD).
2. Experimental Details
2.1. Preparation of the Glass Substrate
The glass substrate of dimensions 75 mm × 75 mm × 1.10 mm was used for deposition of CuS thin film. Substrate were washed with double distilled water and boiled in chromic acid for 2 hours. Further they were washed with detergent and rinsed in acetone with ultrasonic treatment. This process is necessary for substrate cleaning, which creates nucleation centers required in thin film deposition.
2.2. Preparation of CuS Thin Films
CBD is simple and promising method used in CuS thin film preparation. It requires low processing temperature and possibility for large scale deposition. Copper sulphate (CuSO4∙5H2O) was used as a copper source and thiourea (SC(NH2)2) as a sulphur source. Reaction bath contains 10 ml 0.1 M CuSO4・5H2O, 11 ml of 25% aq. ammonia, 10 ml of 0.1 M SC(NH2)2 in 100 ml beaker and rest distilled water to make the volume 50 ml. The pH value was adjusted at 10, to get uniform thin films of CuS on glass substrates. By several trails, the preparative parameters are optimized, concentration of the reactant solutions (viz. CuSO4・5H2O and SC(NH2)2) 0.1 M, and pH 10. The deposition was allowed to proceed at RT for different time durations. Hydroxyl ions are created by ammonia when it reacts with water. These ions make a reaction with thiourea and ionized sulphur is obtained. Due to attractive forces between positive and negative ions of copper (Cu2+) and sulphur (S2−), small cluster of CuS is formed. These clusters are directly nucleated on the substrate surface and there they grow into islands of the condensed phase. Such islands start to form layers consisting of the CuS molecules and CuS thin film gets deposited on the glass substrate surface. The process of nucleation and growth is schematically shown in the Figure 1. After deposition, the glass microslides were taken out from the bath, washed with de-ionised water to separate the loosely bounded CuS atoms, then dried in air. The films of various thicknesses were obtained by varying the deposition time. Deposited thin films of CuS were further studied for various properties. Depositions longer than 70 minutes resulted in peeling of the films from the glass substrates.
2.3. Characterization of CuS Thin Films
The CuS thin films were characterized for structural, morphological, optical and electrical properties. Thickness of CuS thin film is measured by Fizeau fringe technique in which, thickness of thin film is determined as a difference of measured position of interference fringe patterns. When light is incident on the substrate, reflection of light from the surface, interface of film and substrate occurs. Thereby, phase difference will be generated giving interference patterns. The obtained interference pattern can be used for thickness measurement using the following relation:
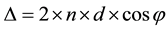 (1)
(1)
where, d―thickness, n―refractive index, φ―refractive angle, Δ―optical path difference.
X-ray diffraction (XRD) patterns of the film were recorded on a Bruker AXS, Germany (D8 Advanced) diffractometer in the scanning range 20˚ - 80˚ (2θ) using CuKα radiations with wavelength 0.15405 nm. S-48500 Type-II (HITACHI HIGH TECHNOLOGY CORPORATION Tokyo, Japan) field emission scanning electron microscope (FESEM) was used for the determination of surface morphology. Using JASCO UV-VIS spectrometer (V-630) the optical properties of CuS thin film were investigated by estimating the absorbance and transmittance relation with wavelength having range 400 nm - 800 nm. The I-V characteristics were studied using Keithley meter (Model No. 2400), over the range from ±2V.
3. Result and Discussion
3.1. Structural Analysis
Figure 2 shows the XRD pattern of CuS thin films of different thickness deposited onto glass substrate. XRD pattern revealed that the CuS thin films are polycrystalline in nature with orthorombic (covellite) crystal structure when matched with JCPDS card No. 78-2122. XRD pattern of the CuS thin films showed sharp peak (113) along with other minor peaks corresponding to (112) and (115) planes. Apart from this a diffraction peak corresponding to 2θ = 38.77˚ was observed, which is a peak arising due to Cu(OH)2 (JCPDS card No. 00-003-0307). Such a phase may originate during the hydrolysis process of Cu2+, which was carried out at RT. Although there is presence of Cu(OH)2, it is found that the crystal structure of CuS has not been much affected. Moreover, the
![]()
Figure 1. Schematic diagram of the chemical synthesis of CuS thin film.
![]()
Figure 2. XRD patterns of CuS thin films deposited with different thickness.
presence of a number of peaks corresponding to CuS indicates that the films are polycrystalline with preferred orientation along (113) plane. It was also seen from the XRD pattern that the intensity of the peak increases with increase in thickness, while no significant shift in the peak position was observed. In fact there is a near-linear dependence of (113) peak intensity with respect to thickness found (see Figure 3). This is quite natural due the fact that with increase in thickness there increases the density of CuS material. For the high intense peak i.e., (113) plane, we calculated the crystallite size using Scherrer’s formula as given below,
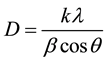 (2)
(2)
where: λ is wavelength of X-ray; β is broadening of diffraction line measured at full width at half of the peak maxima in radians; k is a constant (0.94) and θ is Bragg’s angle. We found that the crystallite size ranges between 30 - 34 nm for all the samples, while there was no specific dependence found with respect to thickness.
3.2. Surface Morphology and Compositional Analysis
Figures 4(a)-(e) show the FESEM images for different thickness of CuS thin film. It is observed from these images that all the CuS thin films have uniform distribution of grains over the surface and mostly these grains fall in nanometer regime. When thickness of the film increases, morphology turned from tiny nanoparticle-like to heavy granules. Furthermore some CuS nanorods were found at Figure 4(a). Considering these, it is very likely that the CuS nanoparticles were nucleated and grown at low thickness and further started to agglomerate to form larger granules with progress of chemical deposition as is seen in the Figures 4(a)-(e). Significantly, microscopic defects like voids, pinholes or peeling are not observed. It can be seen that the thin film deposited at RT is uniform, dense and well adhered to the glass substrate. This kind of dense and compact morphology is essential for the applications of thin film solar cells. Since the leakage of photo-current can be prevented [17] . Higher magnification FESEM image of CuS thin film corresponding to 305 nm thickness indicated that the deposited film is uniformly covered with compact globular structures composed of spherical particles as shown in inset of Figure 4(e). The average diameter of the globule is less than 150 nm as compared with the diameter of the spherical particles (25 - 50 nm), by which the globules are made.
3.3. Optical Analysis
The transmittance spectra of deposited CuS thin films having different thicknesses are shown in Figure 5. It is seen from Figure 5 that the transmittance edge gets shifted toward the longer wavelength, while there is decrease in transmittance (except for 210 nm thickness CuS film after 650 nm wavelength) with the increase in thickness of the CuS thin film. The shifting in the edge may occur due to the diffraction losses appeared as a result of agglomeration of the film thickness. This might also result in change of the refractive index of the material that may vary between the mean values [18] . Further to obtain the band gap values, we used the theory of optical absorption, which gives the relation between the absorption coefficient “α” and the photon energy “hν”, for direct allowed transition as [19] ,
![]()
Figure 3. XRD peak intensities for (113) plane of CuS thin films with respect to film thickness. Note: The line used is to show near-linear dependence.
![]()
Figure 4. FESEM images of CuS thin films of different thickness on glass substrate: (a) 120 nm; (b) 175 nm; (c) 210 nm; (d) 250 nm; and (e) 305 nm (inset of Figure 3(e) shows the higher magnification FESEM image).
![]()
Figure 5. Transmittance spectra of CuS thin films of different thickness on glass substrate.
 (3)
(3)
where “hν” is the photon energy, “Eg” is the optical band gap, “A” is a constant.
Using the above relation, we calculated (αhν)2 and hν values, which is plotted as shown in Figure 6. After extrapolation of the linear portion to the energy axis i.e., x-axis, will yield the optical band gap value of the material. The optical band gap value Eg calculated is found to be decreased from 2.56 eV to 2.42 eV with the increase in the thickness of CuS thin films i.e., from 120 nm to 305 nm, respectively. This may be due to the strong quantum confinement occurring along the thickness direction, so the geometric parameter in CuS enables to reduce the optical band gap in two dimensional quantum controls with high degree of precession [20] . This indicates that the thickness has a strong dependence i.e., inverse dependence on the optical bang gap value of the CuS thin film.
3.4. Photosensitivity Studies
Study of I-V characterization is done in dark and by varying illumination intensities of light onto different thickness CuS thin films [see Figures 7(a)-(e)]. For studying the photosensor properties, an area of 1 cm2 of CuS thin film were selected and silver paste was applied (two Ag contacts separated by a distance of 1 cm) to ensure the good neutral electrical contact. Before light illumination, the I-V curve in dark was measured and then subsequently after light illumination (having different intensities) the corresponding I-V curve was measured. It was seen from all the I-V curves [see Figures 7(a)-(e)], that there is a linear dependence between I-V characteristics indicating ohmic nature of the CuS thin films. Furthermore, a general trend for all CuS samples showed that with increase in the illumination intensity, the photoresistance decreases (see Figure 8). This suggests that the increase in the incident photon energy breaks some of the covalent bonds in the CuS semiconductor and as a result generates free electron-hole pairs (EHP), which becomes available for current conduction thereby decreasing the resistance [21] [22] . Notwithstanding to this, it was also found that the increase in thickness of the CuS films, decreases the photoresistance values (see Figure 8). Significantly, it was observed for CuS films of 210 nm thickness that there is an uneven change in the photoresistance value with increase in the illumination intensity (see the orange square indicated in inset of Figure 8). As such with illumination of 5800 Lux intensity, the photoresistance was found to be increased and later it again started to decrease with increase in intensity. These uneven changes of the photoresistance with illumination intensity can be a direct consequence of the transmittance properties seen for this particular film. The transmittance was suddenly increased for this film as compared to other thickness films, indicating that at certain value of illuminated light intensity the films becomes transparent to the light and the possible EHP is hindered thereby causing an increase in the photoresistance values. This study suggests that though the light illumination intensity can increase the number of EHPs; but one can possibly have control over this number by simply controlling the thickness of the CuS thin film. In addition, it indicates the suitability of CuS thin films as smart photosensor devices.
![]()
Figure 6. Plot of (αhν)2 versus hν for CuS thin films of different thicknesses.
![]()
Figure 7. Current-voltage (I-V) characteristics curve for CuS thin film taken in dark as well as with different illumination intensities for: (a) 120 nm; (b) 175 nm; (c) 210 nm; (d) 250 nm and (e) 305 nm.
![]()
Figure 8. Plot of photo-resistance versus thickness taken at different illumination intensities. Inset shows the magnified view, while the orange square indicates the uneven variation of photoresistance for particular 210 nm CuS thick films.
4. Conclusion
CuS thin films with different thickness have been successfully deposited by the simple and cost-effective chemical bath deposition technique. Increase in the thickness of the CuS films increased the crystallinity as well as morphological properties. Dense morphology with globular structures was confirmed for the large thickness CuS samples. Uneven changes in the transmittance helped us to understand the photo-resistance properties of the CuS thin films. Significant influence of the thickness on the photoresistance values was confirmed indicating that one could manipulate the photoresistance by simply controlling the thickness of the CuS thin films.
Acknowledgements
The authors are thankful to Head, UDCT Department, North Maharashtra University Jalgaon and Principal, Dr. P. V. Ramaiah, C. H. C. Arts, S. G. P. Commerce, and B. B. J. P. Science College, Taloda, for providing the laboratory facilities. SRG thank Chairman and Management members of Arts and Commerce College Trusts for constant encouragement for doing research work. NGD thanks Department of Science and Technology (DST) for awarding DST Inspire Faculty Award [IFA-PH-61/01/08/2013].
NOTES
*Corresponding authors.